Deconstructing voltage sensor function and pharmacology in sodium channels
- PMID: 19005548
- PMCID: PMC2587061
- DOI: 10.1038/nature07473
Deconstructing voltage sensor function and pharmacology in sodium channels
Abstract
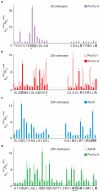
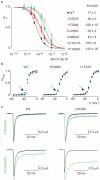
Voltage-activated sodium (Na(v)) channels are crucial for the generation and propagation of nerve impulses, and as such are widely targeted by toxins and drugs. The four voltage sensors in Na(v) channels have distinct amino acid sequences, raising fundamental questions about their relative contributions to the function and pharmacology of the channel. Here we use four-fold symmetric voltage-activated potassium (K(v)) channels as reporters to examine the contributions of individual S3b-S4 paddle motifs within Na(v) channel voltage sensors to the kinetics of voltage sensor activation and to forming toxin receptors. Our results uncover binding sites for toxins from tarantula and scorpion venom on each of the four paddle motifs in Na(v) channels, and reveal how paddle-specific interactions can be used to reshape Na(v) channel activity. One paddle motif is unique in that it slows voltage sensor activation, and toxins selectively targeting this motif impede Na(v) channel inactivation. This reporter approach and the principles that emerge will be useful in developing new drugs for treating pain and Na(v) channelopathies.
Figures




Comment in
-
Ion channels: The voltage-sensor quartet.Nature. 2008 Nov 13;456(7219):183-5. doi: 10.1038/456183a. Nature. 2008. PMID: 19005542 Free PMC article.
References
-
- Frech GC, VanDongen AM, Schuster G, Brown AM, Joho RH. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989;340:642–5. - PubMed
-
- Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987;237:770–5. - PubMed
-
- Auld VJ, et al. A rat brain Na+ channel alpha subunit with novel gating properties. Neuron. 1988;1:449–61. - PubMed
-
- Trimmer JS, et al. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989;3:33–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

