Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts
- PMID: 19006178
- PMCID: PMC2706022
- DOI: 10.1002/jcp.21623
Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts
Abstract
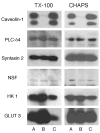
Despite enormous interest in membrane raft micro-domains, no studies in any cell type have defined the relative compositions of the raft fractions on the basis of their major components--sterols, phospholipids, and proteins--or additional raft-associating lipids such as the ganglioside, G(M1). Our previous localization data in live sperm showed that the plasma membrane overlying the acrosome represents a stabilized platform enriched in G(M1) and sterols. These findings, along with the physiological requirement for sterol efflux for sperm to function, prompted us to characterize sperm membrane fractions biochemically. After confirming limitations of commonly used detergent-based approaches, we utilized a non-detergent-based method, separating membrane fractions that were reproducibly distinct based on sterol, G(M1), phospholipid, and protein compositions (both mass amounts and molar ratios). Based on fraction buoyancy and biochemical composition, we identified at least three highly reproducible sub-types of membrane raft. Electron microscopy revealed that raft fractions were free of visible contaminants and were separated by buoyancy rather than morphology. Quantitative proteomic comparisons and fluorescence localization of lipids suggested that different organelles contributed differentially to individual raft sub-types, but that multiple membrane micro-domain sub-types could exist within individual domains. This has important implications for scaffolding functions broadly associated with rafts. Most importantly, we show that the common practice of characterizing membrane domains as either "raft" or "non-raft" oversimplifies the actual biochemical complexity of cellular membranes.
Figures

Similar articles
-
Mechanisms underlying the micron-scale segregation of sterols and GM1 in live mammalian sperm.J Cell Physiol. 2009 Mar;218(3):522-36. doi: 10.1002/jcp.21624. J Cell Physiol. 2009. PMID: 19012288 Free PMC article.
-
Characterization of the proteomes associating with three distinct membrane raft sub-types in murine sperm.Proteomics. 2010 Oct;10(19):3494-505. doi: 10.1002/pmic.201000002. Proteomics. 2010. PMID: 20815087 Free PMC article.
-
Segregation of micron-scale membrane sub-domains in live murine sperm.J Cell Physiol. 2006 Mar;206(3):636-46. doi: 10.1002/jcp.20504. J Cell Physiol. 2006. PMID: 16222699
-
Lipid raft proteomics: more than just detergent-resistant membranes.Subcell Biochem. 2007;43:35-47. doi: 10.1007/978-1-4020-5943-8_4. Subcell Biochem. 2007. PMID: 17953390 Review.
-
Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications.Subcell Biochem. 2004;37:167-215. doi: 10.1007/978-1-4757-5806-1_5. Subcell Biochem. 2004. PMID: 15376621 Review.
Cited by
-
Phospholipase B is activated in response to sterol removal and stimulates acrosome exocytosis in murine sperm.J Biol Chem. 2013 Sep 27;288(39):28104-15. doi: 10.1074/jbc.M113.450981. Epub 2013 Aug 13. J Biol Chem. 2013. PMID: 23943622 Free PMC article.
-
Ion channels, phosphorylation and mammalian sperm capacitation.Asian J Androl. 2011 May;13(3):395-405. doi: 10.1038/aja.2010.69. Asian J Androl. 2011. PMID: 21540868 Free PMC article. Review.
-
Lipid bilayer composition affects transmembrane protein orientation and function.J Lipids. 2011;2011:208457. doi: 10.1155/2011/208457. Epub 2011 Jan 26. J Lipids. 2011. PMID: 21490797 Free PMC article.
-
Mice lacking FABP9/PERF15 develop sperm head abnormalities but are fertile.Dev Biol. 2010 Dec 15;348(2):177-89. doi: 10.1016/j.ydbio.2010.09.019. Epub 2010 Oct 20. Dev Biol. 2010. PMID: 20920498 Free PMC article.
-
Bicarbonate induces membrane reorganization and CBR1 and TRPV1 endocannabinoid receptor migration in lipid microdomains in capacitating boar spermatozoa.J Membr Biol. 2010 Dec;238(1-3):33-41. doi: 10.1007/s00232-010-9316-8. Epub 2010 Nov 23. J Membr Biol. 2010. PMID: 21104238
References
-
- Bou Khalil M, Chakrabandhu K, Xu H, Weerachatyanukul W, Buhr M, Berger T, Carmona E, Vuong N, Kumarathasan P, Wong PT, Carrier D, Tanphaichitr N. Sperm capacitation induces an increase in lipid rafts having zona pellucida binding ability and containing sulfogalactosylglycerolipid. Dev Biol. 2006;290(1):220–235. - PubMed
-
- Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 2006;21:430–439. - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68(3):533–544. - PubMed
-
- Burgos MH, Gutierrez LS. The Golgi complex of the early spermatid in guinea pig. The Anatomical record. 1986;216(2):139–145. - PubMed
-
- Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS. A novel function for CRISP1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biol Reprod. 2007;77(5):848–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

