Secondary motoneurons in juvenile and adult zebrafish: axonal pathfinding errors caused by embryonic nicotine exposure
- PMID: 19006183
- PMCID: PMC2798059
- DOI: 10.1002/cne.21903
Secondary motoneurons in juvenile and adult zebrafish: axonal pathfinding errors caused by embryonic nicotine exposure
Abstract
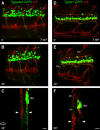
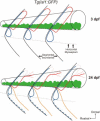
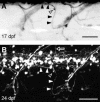
Nicotine is a drug of abuse that has been reported to have many adverse effects on the developing nervous system. We previously demonstrated that embryonic exposure to nicotine alters axonal pathfinding of spinal secondary motoneurons in zebrafish. We hypothesize that these changes will persist into adulthood. The Tg(isl1:GFP) line of zebrafish, which expresses green fluorescent protein (GFP) in a subtype of spinal secondary motoneurons, was used to investigate potential long-term consequences of nicotine exposure on motoneuron development. Anatomical characterization of Tg(isl1:GFP) zebrafish ranging between 3 and 30 days postfertilization (dpf) was initially performed in fixed tissue to characterize axonal trajectories in larval and juvenile fish. Tg(isl1:GFP) embryos were transiently exposed to 5-30 microM nicotine. They were then rescued from nicotine and raised into later stages of life (3-30 dpf) and fixed for microscopic examination. Morphological analysis revealed that nicotine-induced abnormalities in secondary motoneuron anatomy were still evident in juvenile fish. Live imaging of Tg(isl1:GFP) zebrafish using fluorescent stereomicroscopy revealed that the nicotine-induced changes in motoneuron axonal pathfinding persisted into adulthood. We detected abnormalities in 37-dpf fish that were transiently exposed to nicotine as embryos. These fish were subsequently imaged over a 7-week period of time until they were approximately 3 months of age. These pathfinding errors of spinal secondary motoneuron axons detected at 37 dpf persisted within the same fish until 86 dpf, the latest age analyzed. These findings indicate that exposure to nicotine during embryonic development can have permanent consequences for motoneuron anatomy in zebrafish.
Figures









References
-
- Beattie CE, Hatta K, Halpern ME, Liu H, Eisen JS, Kimmel CB. Temporal separation in the specification of primary and secondary motoneurons in zebrafish. Dev Biol. 1997;187:171–182. - PubMed
-
- Bhatt DH, Otto SJ, Depoister B, Fetcho JR. Cyclic AMP-induced repair of zebrafish spinal circuits. Science. 2004;305:254–258. - PubMed
-
- Burns FR, von Kannen S, Guy L, Raper JA, Kamholz J, Chang S. DM-GRASP, a novel immunoglobulin superfamily axonal surface protein that supports neurite extension. Neuron. 1991;7:209–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

