Analysis of the reaction of carbachol with acetylcholinesterase using thioflavin T as a coupled fluorescence reporter
- PMID: 19006330
- PMCID: PMC2655144
- DOI: 10.1021/bi8015197
Analysis of the reaction of carbachol with acetylcholinesterase using thioflavin T as a coupled fluorescence reporter
Abstract
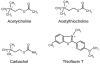
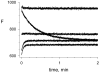
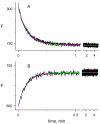
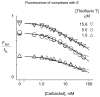
Acetylcholinesterase (AChE) contains a narrow and deep active site gorge with two sites of ligand binding, an acylation site (or A-site) at the base of the gorge and a peripheral site (or P-site) near the gorge entrance. The P-site contributes to catalytic efficiency by transiently binding substrates on their way to the acylation site, where a short-lived acylated enzyme intermediate is produced. Carbamates are very poor substrates that, like other AChE substrates, form an initial enzyme-substrate complex with free AChE (E) and proceed to an acylated enzyme intermediate (EC), which is then hydrolyzed. However, the hydrolysis of EC is slow enough to resolve the acylation and deacylation steps on the catalytic pathway. Here, we focus on the reaction of carbachol (carbamoylcholine) with AChE. The kinetics and thermodynamics of this reaction are of special interest because carbachol is an isosteric analogue of the physiological substrate acetylcholine. We show that the reaction can be monitored with thioflavin T as a fluorescent reporter group. The fluorescence of thioflavin T is strongly enhanced when it binds to the P-site of AChE, and this fluorescence is partially quenched when a second ligand binds to the A-site to form a ternary complex. Analysis of the fluorescence reaction profiles was challenging because four thermodynamic parameters and two fluorescence coefficients were fitted from the combined data both for E and for EC. Respective equilibrium dissociation constants of 6 and 26 mM were obtained for carbachol binding to the A- and P-sites in E and of 2 and 32 mM for carbachol binding to the A- and P-sites in EC. These constants for the binding of carbachol to the P-site are about an order of magnitude larger (i.e., indicating lower affinity) than previous estimates for the binding of acetylthiocholine to the P-site.
Figures
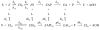
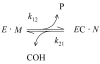
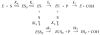

Similar articles
-
Molecular basis of inhibition of substrate hydrolysis by a ligand bound to the peripheral site of acetylcholinesterase.Chem Biol Interact. 2010 Sep 6;187(1-3):135-41. doi: 10.1016/j.cbi.2010.05.009. Epub 2010 May 21. Chem Biol Interact. 2010. PMID: 20493829 Free PMC article.
-
Monitoring the reaction of carbachol with acetylcholinesterase by thioflavin T fluorescence and acetylthiocholine hydrolysis.Chem Biol Interact. 2008 Sep 25;175(1-3):235-41. doi: 10.1016/j.cbi.2008.06.002. Epub 2008 Jun 17. Chem Biol Interact. 2008. PMID: 18602908 Free PMC article.
-
Unmasking tandem site interaction in human acetylcholinesterase. Substrate activation with a cationic acetanilide substrate.Biochemistry. 2003 May 13;42(18):5438-52. doi: 10.1021/bi027065u. Biochemistry. 2003. PMID: 12731886
-
Heterocyclic inhibitors of AChE acylation and peripheral sites.Farmaco. 2005 Jun-Jul;60(6-7):465-73. doi: 10.1016/j.farmac.2005.03.010. Farmaco. 2005. PMID: 15878569 Review.
-
A preliminary comparison of structural models for catalytic intermediates of acetylcholinesterase.Chem Biol Interact. 1999 May 14;119-120:43-52. doi: 10.1016/s0009-2797(99)00012-5. Chem Biol Interact. 1999. PMID: 10421437 Review.
Cited by
-
Hopeahainol A binds reversibly at the acetylcholinesterase (AChE) peripheral site and inhibits enzyme activity with a novel higher order concentration dependence.Chem Biol Interact. 2016 Nov 25;259(Pt B):78-84. doi: 10.1016/j.cbi.2016.05.032. Epub 2016 Jun 11. Chem Biol Interact. 2016. PMID: 27297626 Free PMC article.
-
Selective Targeting of Extracellular Insulin-Degrading Enzyme by Quasi-Irreversible Thiol-Modifying Inhibitors.ACS Chem Biol. 2015 Dec 18;10(12):2716-24. doi: 10.1021/acschembio.5b00334. Epub 2015 Sep 30. ACS Chem Biol. 2015. PMID: 26398879 Free PMC article.
-
ADME profiling, molecular docking, DFT, and MEP analysis reveal cissamaline, cissamanine, and cissamdine from Cissampelos capensis L.f. as potential anti-Alzheimer's agents.RSC Adv. 2024 Mar 25;14(14):9878-9891. doi: 10.1039/d4ra01070a. eCollection 2024 Mar 20. RSC Adv. 2024. PMID: 38528929 Free PMC article.
-
Molecular basis of inhibition of substrate hydrolysis by a ligand bound to the peripheral site of acetylcholinesterase.Chem Biol Interact. 2010 Sep 6;187(1-3):135-41. doi: 10.1016/j.cbi.2010.05.009. Epub 2010 May 21. Chem Biol Interact. 2010. PMID: 20493829 Free PMC article.
-
Rate-limiting step in the decarbamoylation of acetylcholinesterases with large carbamoyl groups.Chem Biol Interact. 2019 Aug 1;308:392-395. doi: 10.1016/j.cbi.2019.06.004. Epub 2019 Jun 6. Chem Biol Interact. 2019. PMID: 31175846 Free PMC article. Review.
References
-
- De Ferrari GV, Mallender WD, Inestrosa NC, Rosenberry TL. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J. Biol. Chem. 2001;276:23282–23287. - PubMed
-
- Johnson JL, Cusack B, Davies MP, Fauq A, Rosenberry TL. Unmasking tandem site interaction in human acetylcholinesterase. Substrate activation with a cationic acetanilide substrate. Biochemistry. 2003;42:5438–5452. - PubMed
-
- Brufani M, Lippa S, Marta M, Oradei A, Pomponi M. Acetylcholinesterase inhibition by eserine: rate constants of reaction. Part II. Ital. J. Biochem. 1985;34:328–340. - PubMed
-
- Rosenberry TL, Johnson JL, Cusack B, Thomas J, Emani S, Venkatasubban KS. Interactions between the peripheral site and the acylation site in acetylcholinesterase. Chem. Biol. Interact. 2005;157-158:181–189. - PubMed
-
- Rosenberry TL. Acetylcholinesterase. In: Meister A, editor. Advances in Enzymology. John Wiley & Sons; New York: 1975. pp. 103–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

