Total synthesis of (+)-azaspiracid-1. An exhibition of the intricacies of complex molecule synthesis
- PMID: 19006391
- PMCID: PMC3408805
- DOI: 10.1021/ja804659n
Total synthesis of (+)-azaspiracid-1. An exhibition of the intricacies of complex molecule synthesis
Abstract
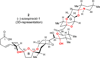
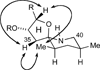
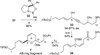
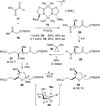
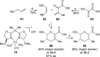
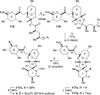
The synthesis of the marine neurotoxin azaspiracid-1 has been accomplished. The individual fragments were synthesized by catalytic enantioselective processes: A hetero-Diels-Alder reaction to afford the E- and HI-ring fragments, a carbonyl-ene reaction to furnish the CD-ring fragment, and a Mukaiyama aldol reaction to deliver the FG-ring fragment. The subsequent fragment couplings were accomplished by aldol and sulfone anion methodologies. All ketalization events to form the nonacyclic target were accomplished under equilibrating conditions utilizing the imbedded configurations of the molecule to adopt one favored conformation. A final fragment coupling of the anomeric EFGHI-sulfone anion to the ABCD-aldehyde completed the convergent synthesis of (+)-azaspiracid-1.
Figures

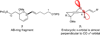















References
-
- McMahon T, Silke J. Harmful Algae News. 1996;14:2.
-
- James KJ, Furey A, Lehane M, Ramstad H, Aune T, Hovgaard P, Morris S, Higman W, Satake M, Yasumoto T. Toxicon. 2002;40:909–915. - PubMed
-
- Magdalena AB, Lehane M, Krys S, Fernandez ML, Furey A, James KJ. Toxicon. 2003;42:105–108. - PubMed
-
- James KJ, Moroney C, Roden C, Satake M, Yasumoto T, Lehane M, Furey A. Toxicon. 2003;41:145–151. - PubMed
-
- Taleb H, Vale P, Amanhir R, Benhadouch A, Sagou R, Chafik A. J. Shellfish Res. 2006;25:1067–1070.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

