Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel
- PMID: 19006400
- PMCID: PMC2593885
- DOI: 10.1021/cr8004422
Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel
Figures












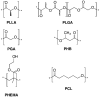
References
-
- Lowenstam HA. Science. 1981;211:1126. - PubMed
-
- Mann S. Nature. 1993;365:499.
-
- Mann S, Archibald DD, Didymus JM, Douglas T, Heywood BR, Meldrum FC, Reeves NJ. Science. 1993;261:1286. - PubMed
-
- Fritz M, Belcher AM, Radmacher M, Walters DA, Hansma PK, Stucky GD, Morse DE, Mann S. Nature. 1994;371:49.
-
- Shen X, Belcher AM, Hansma PK, Stucky GD, Morse DE. J. Biol. Chem. 1997;272:32472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

