Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells
- PMID: 19006694
- PMCID: PMC3140207
- DOI: 10.1016/j.immuni.2008.09.018
Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells
Abstract
CD4(+)Foxp3(+) regulatory T (Treg) cells originate primarily from thymic differentiation, but conversion of mature T lymphocytes to Foxp3 positivity can be elicited by several means, including in vitro activation in the presence of TGF-beta. Retinoic acid (RA) increases TGF-beta-induced expression of Foxp3, through unknown molecular mechanisms. We showed here that, rather than enhancing TGF-beta signaling directly in naive CD4(+) T cells, RA negatively regulated an accompanying population of CD4(+) T cells with a CD44(hi) memory and effector phenotype. These memory cells actively inhibited the TGF-beta-induced conversion of naive CD4(+) T cells through the synthesis of a set of cytokines (IL-4, IL-21, IFN-gamma) whose expression was coordinately curtailed by RA. This indirect effect was evident in vivo and required the expression of the RA receptor alpha. Thus, cytokine-producing CD44(hi) cells actively restrain TGF-beta-mediated Foxp3 expression in naive T cells, and this balance can be shifted or fine-tuned by RA.
Figures
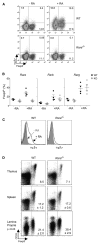




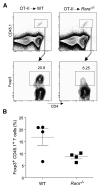
Comment in
-
Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells.Immunity. 2009 Apr 17;30(4):471-2; author reply 472-3. doi: 10.1016/j.immuni.2009.03.008. Immunity. 2009. PMID: 19371709 Free PMC article. No abstract available.
References
-
- Abb J, Abb H, Deinhardt F. Effect of retinoic acid on the spontaneous and interferon-induced activity of human natural killer cells. Int J Cancer. 1982a;30:307–310. - PubMed
-
- Abb J, Abb H, Deinhardt F. Retinoic acid suppression of human leukocyte interferon production. Immunopharmacology. 1982b;4:303–310. - PubMed
-
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

