Synthetic and crystallographic studies of a new inhibitor series targeting Bacillus anthracis dihydrofolate reductase
- PMID: 19007108
- PMCID: PMC2645930
- DOI: 10.1021/jm800776a
Synthetic and crystallographic studies of a new inhibitor series targeting Bacillus anthracis dihydrofolate reductase
Abstract
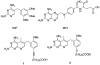
Bacillus anthracis, the causative agent of anthrax, poses a significant biodefense danger. Serious limitations in approved therapeutics and the generation of resistance have produced a compelling need for new therapeutic agents against this organism. Bacillus anthracis is known to be insensitive to the clinically used antifolate, trimethoprim, because of a lack of potency against the dihydrofolate reductase enzyme. Herein, we describe a novel lead series of B. anthracis dihydrofolate reductase inhibitors characterized by an extended trimethoprim-like scaffold. The best lead compound adds only 22 Da to the molecular weight and is 82-fold more potent than trimethoprim. An X-ray crystal structure of this lead compound bound to B. anthracis dihydrofolate reductase in the presence of NADPH was determined to 2.25 A resolution. The structure reveals several features that can be exploited for further development of this lead series.
Figures



References
-
- Mock M, Fouet A. Anthrax. Annu. Rev. Microbiol. 2001;55:647–671. - PubMed
-
- Davidson R, Cavalcanti R, Brunton J, Bast D, DeAzavedo J, Kibsey P, Fleming C, Low D. Resistance to levofloxacin and failure of treatment of Pneumococcal pneumonia. N. Engl. J. Med. 2002;346:747–750. - PubMed
-
- Neuhauser M, Weinstein R, Rydman R, Danziger L, Karam G, Quinn J. Antibiotic resistance among gram-negative Bacilli in US intensive care units. JAMA, J. Am. Med. Assoc. 2003;289:885–888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

