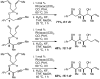
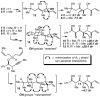
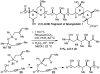
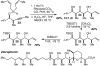Tandem silylformylation-crotylsilylation/Tamao oxidation of internal alkynes: a remarkable example of generating complexity from simplicity
- PMID: 19007175
- PMCID: PMC2628290
- DOI: 10.1021/ol802489w
Tandem silylformylation-crotylsilylation/Tamao oxidation of internal alkynes: a remarkable example of generating complexity from simplicity
Abstract
The rhodium-catalyzed tandem silylformylation-crotylsilylation reaction has been extended to include internal alkynes. Tamao oxidation of the initial product leads to the production of a substituted enol, which undergoes highly diastereoselective tautomerization. The resulting one-pot procedure fashions three new stereocenters, a ketone, and a terminal alkene from a butenyl group, a propynyl group, a silyl hydride, H2O2, and CO.
Figures
Similar articles
-
An "aprotic" Tamao oxidation/syn-selective tautomerization reaction for the efficient synthesis of the C1-C9 fragment of fludelone.Org Lett. 2012 Sep 21;14(18):4890-3. doi: 10.1021/ol302221s. Epub 2012 Sep 5. Org Lett. 2012. PMID: 22950417 Free PMC article.
-
Desymmetrization of 4-dimethylsiloxy-1,6-heptadiynes through sequential double silylformylation.Org Lett. 2001 May 3;3(9):1303-5. doi: 10.1021/ol0156594. Org Lett. 2001. PMID: 11348220
-
Tandem intramolecular silylformylation-crotylsilylation: highly efficient synthesis of polyketide fragments.J Am Chem Soc. 2002 Jul 10;124(27):7890-1. doi: 10.1021/ja026511y. J Am Chem Soc. 2002. PMID: 12095319
-
Beyond the Roche ester: a new approach to polypropionate stereotriad synthesis.Org Lett. 2014 Feb 21;16(4):1180-3. doi: 10.1021/ol500051e. Epub 2014 Feb 6. Org Lett. 2014. PMID: 24502345 Free PMC article.
-
Rhodium-Catalyzed (5 + 2) and (5 + 1) Cycloadditions Using 1,4-Enynes as Five-Carbon Building Blocks.Acc Chem Res. 2020 Jan 21;53(1):231-243. doi: 10.1021/acs.accounts.9b00477. Epub 2019 Dec 10. Acc Chem Res. 2020. PMID: 31820914 Free PMC article. Review.
Cited by
-
Synthesis and Evaluation of a Linkable Functional Group-Equipped Analogue of the Epothilones.ACS Med Chem Lett. 2017 May 1;8(7):701-704. doi: 10.1021/acsmedchemlett.7b00131. eCollection 2017 Jul 13. ACS Med Chem Lett. 2017. PMID: 28740601 Free PMC article.
-
Local Desymmetrization through Diastereotopic Group Selection: An Enabling Strategy for Natural Product Synthesis.European J Org Chem. 2017 Mar 17;2017(11):1381-1390. doi: 10.1002/ejoc.201601481. Epub 2017 Feb 7. European J Org Chem. 2017. PMID: 28533701 Free PMC article.
-
Complex fragment coupling by crotylation: A powerful tool for polyketide natural product synthesis.Chem Sci. 2012 Nov 1;3(11):3326-3330. doi: 10.1039/C2SC21325G. Chem Sci. 2012. PMID: 25165502 Free PMC article.
-
A highly step-economical synthesis of dictyostatin.Angew Chem Int Ed Engl. 2013 Jun 24;52(26):6757-61. doi: 10.1002/anie.201302565. Epub 2013 May 10. Angew Chem Int Ed Engl. 2013. PMID: 23666786 Free PMC article. No abstract available.
-
A more comprehensive and highly practical solution to enantioselective aldehyde crotylation.J Am Chem Soc. 2011 May 4;133(17):6517-20. doi: 10.1021/ja200712f. Epub 2011 Apr 12. J Am Chem Soc. 2011. PMID: 21486033 Free PMC article.
References
-
- O'Malley SJ, Leighton JL. Angew Chem Int Ed. 2001;40:2915. - PubMed
- Zacuto MJ, O'Malley SJ, Leighton JL. J Am Chem Soc. 2002;124:7890. - PubMed
- Zacuto MJ, O'Malley SJ, Leighton JL. Tetrahedron. 2003;59:8889.
- Schmidt DR, Park PK, Leighton JL. Org Lett. 2003;5:3535. - PubMed
- Park PK, O'Malley SJ, Schmidt DR, Leighton JL. J Am Chem Soc. 2006;128:2796. - PMC - PubMed
-
-
For seminal early examples of silylformylation of internal alkynes, see: Matsuda I, Ogiso A, Sato S, Izumi Y. J Am Chem Soc. 1989;111:2332.Doyle MP, Shanklin MS. Organometallics. 1994;13:1081.Monteil F, Matsuda I, Alper H. J Am Chem Soc. 1995;117:4419.Ojima I, Vidal E, Tzamarioudaki M, Matsuda I. J Am Chem Soc. 1995;117:6797.
-
-
- Tamao K, Kumada H. Tetrahedron Lett. 1984;25:321.
- Jones GR, Landais Y. Tetrahedron. 1996;52:7599.
-
-
For an example of a Tamao oxidation of a vinylsilane that generates a prochiral enol that undergoes a diastereoselective tautomerization, see: Suginome M, Matsunaga SI, Ito Y. Synlett. 1995:941.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources