New nitrosyl derivatives of diiron dithiolates related to the active site of the [FeFe]-hydrogenases
- PMID: 19007207
- PMCID: PMC2736309
- DOI: 10.1021/ic801542w
New nitrosyl derivatives of diiron dithiolates related to the active site of the [FeFe]-hydrogenases
Abstract
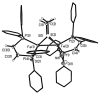
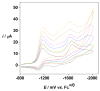
Nitrosyl derivatives of diiron dithiolato carbonyls have been prepared starting from the precursor Fe(2)(S(2)C(n)H(2n))(dppv)(CO)(4) (dppv = cis-1,2-bis(diphenylphosphinoethylene). These studies expand the range of substituted diiron(I) dithiolato carbonyl complexes. From [Fe(2)(S(2)C(2)H(4))(CO)(3)(dppv)(NO)]BF(4) ([1(CO)(3)]BF(4)), the following compounds were prepared: [1(CO)(2)(PMe(3))]BF(4), [1(CO)(dppv)]BF(4), NEt(4)[1(CO)(CN)(2)], and 1(CO)(CN)(PMe(3)). Some of these substitution reactions occur via the addition of 2 equiv of the nucleophile followed by the dissociation of one nucleophile and decarbonylation. Such a double adduct was characterized crystallographically in the case of [Fe(2)(S(2)C(2)H(4))(CO)(3)(dppv)(NO)(PMe(3))(2)]BF(4). This result shows that the addition of two ligands causes scission of the Fe-Fe bond and one Fe-S bond. When cyanide is the nucleophile, nitrosyl migrates away from the Fe(dppv) site, yielding a Fe(CN)(2)(NO) derivative. Compounds [1(CO)(3)]BF(4), [1(CO)(2)(PMe(3))]BF(4), and [1(CO)(dppv)]BF(4) were also prepared by the addition of NO(+) to the di-, tri-, and tetrasubstituted precursors. In these cases, the NO(+) appears to form an initial 36e(-) adduct containing terminal Fe-NO, followed by decarbonylation. Several complexes were prepared by the addition of NO to the mixed-valence Fe(I)Fe(II) derivatives. The diiron nitrosyl complexes reduce at mild potentials and in certain cases form weak adducts with CO. IR and EPR spectra of 1(CO)(dppv), generated by low-temperature reduction of [1(CO)(dppv)]BF(4) with Co(C(5)Me(5))(2), indicates that the SOMO is located on the FeNO subunit.
Figures
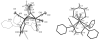







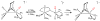
Similar articles
-
Redox and structural properties of mixed-valence models for the active site of the [FeFe]-hydrogenase: progress and challenges.Inorg Chem. 2008 Aug 18;47(16):7405-14. doi: 10.1021/ic8007552. Epub 2008 Jul 12. Inorg Chem. 2008. PMID: 18620387 Free PMC article.
-
Nitrosyl derivatives of diiron(I) dithiolates mimic the structure and Lewis acidity of the [FeFe]-hydrogenase active site.J Am Chem Soc. 2008 Sep 10;130(36):12021-30. doi: 10.1021/ja802268p. Epub 2008 Aug 14. J Am Chem Soc. 2008. PMID: 18700771 Free PMC article.
-
Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere.Acc Chem Res. 2015 Jul 21;48(7):2107-16. doi: 10.1021/acs.accounts.5b00177. Epub 2015 Jun 16. Acc Chem Res. 2015. PMID: 26079848 Free PMC article.
-
Connecting [NiFe]- and [FeFe]-hydrogenases: mixed-valence nickel-iron dithiolates with rotated structures.Inorg Chem. 2012 Aug 20;51(16):8931-41. doi: 10.1021/ic300910r. Epub 2012 Jul 27. Inorg Chem. 2012. PMID: 22838645 Free PMC article.
-
Chelate control of diiron(I) dithiolates relevant to the [Fe-Fe]- hydrogenase active site.Inorg Chem. 2007 Mar 5;46(5):1655-64. doi: 10.1021/ic0618706. Epub 2007 Feb 6. Inorg Chem. 2007. PMID: 17279743 Free PMC article.
Cited by
-
Recent Advances in Multinuclear Metal Nitrosyl Complexes.Coord Chem Rev. 2016 Jan 1;306(Pt 2):678-700. doi: 10.1016/j.ccr.2015.03.026. Epub 2015 Apr 16. Coord Chem Rev. 2016. PMID: 26744544 Free PMC article.
-
Reaction of Aryl Diazonium Salts and Diiron(I) Dithiolato Carbonyls: Evidence for Radical Intermediates.Organometallics. 2012 Apr 23;31(8):3447-3450. doi: 10.1021/om300107s. Epub 2012 Mar 29. Organometallics. 2012. PMID: 22962513 Free PMC article.
-
Stereochemistry of electrophilic attack at 34e⁻ dimetallic complexes: the case of diiron dithiolato carbonyls + MeS⁺.Chem Commun (Camb). 2011 Jun 21;47(23):6554-6. doi: 10.1039/c1cc10858a. Chem Commun (Camb). 2011. PMID: 21776614 Free PMC article.
References
-
- Liu X, Ibrahim SK, Tard C, Pickett CJ. Coord Chem Rev. 2005;249:1641–1652.
- Mejia-Rodriguez R, Chong D, Reibenspies JH, Soriaga MP, Darensbourg MY. J Am Chem Soc. 2004;126:12004–12014. - PubMed
- Zhao X, Georgakaki IP, Miller ML, Yarbrough JC, Darensbourg MY. J Am Chem Soc. 2001;123:9710–9711. - PubMed
-
- Vincent KA, Parkin A, Armstrong FA. Chem Rev. 2007;107:4366–4413. - PubMed
-
- Butler AR, Megson IL. Chem Rev. 2002;102:1155–1165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

