The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm
- PMID: 19007407
- PMCID: PMC2741168
- DOI: 10.1111/j.1365-2958.2008.06503.x
The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm
Abstract
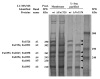
A defining characteristic of the suspected periodontal pathogen Fusobacterium nucleatum is its ability to adhere to a plethora of oral bacteria. This distinguishing feature is suggested to play an important role in oral biofilm formation and pathogenesis, with fusobacteria proposed to serve as central 'bridging organisms' in the architecture of the oral biofilm bringing together species which would not interact otherwise. Previous studies indicate that these bacterial interactions are mediated by galactose- or arginine-inhibitable adhesins although genetic evidence for the role and nature of these proposed adhesins remains elusive. To characterize these adhesins at the molecular level, the genetically transformable F. nucleatum strain ATCC 23726 was screened for adherence properties, and arginine-inhibitable adhesion was evident, while galactose-inhibitable adhesion was not detected. Six potential arginine-binding proteins were isolated from the membrane fraction of F. nucleatum ATCC 23726 and identified via mass spectroscopy as members of the outer membrane family of proteins in F. nucleatum. Inactivation of the genes encoding these six candidates for arginine-inhibitable adhesion and two additional homologues revealed that only a mutant derivative carrying an insertion in Fn1526 (now designated as radD) demonstrated significantly decreased co-aggregation with representatives of the gram-positive 'early oral colonizers'. Lack of the 350 kDa outer membrane protein encoded by radD resulted in the failure to form the extensive structured biofilm observed with the parent strain when grown in the presence of Streptococcus sanguinis ATCC 10556. These findings indicate that radD is responsible for arginine-inhibitable adherence of F. nucleatum and provides definitive molecular evidence that F. nucleatum adhesins play a vital role in inter-species adherence and multispecies biofilm formation.
Figures





References
-
- Edwards AM, Grossman TJ, Rudney JD. Association of a high-molecular weight arginine-binding protein of Fusobacterium nucleatum ATCC 10953 with adhesion to secretory immunoglobulin A and co-aggregation with Streptococcus cristatus. Oral Microbiology and Immunology. 2007;22:217–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

