Role of the UPS in Liddle syndrome
- PMID: 19007435
- PMCID: PMC2582799
- DOI: 10.1186/1471-2091-9-S1-S5
Role of the UPS in Liddle syndrome
Abstract
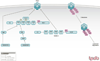
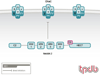
Hypertension is a serious medical problem affecting a large population worldwide. Liddle syndrome is a hereditary form of early onset hypertension caused by mutations in the epithelial Na+ channel (ENaC). The mutated region, called the PY (Pro-Pro-x-Tyr) motif, serves as a binding site for Nedd4-2, an E3 ubiquitin ligase from the HECT family. Nedd4-2 binds the ENaC PY motif via its WW domains, normally leading to ENaC ubiquitylation and endocytosis, reducing the number of active channels at the plasma membrane. In Liddle syndrome, this endocytosis is impaired due to the inability of the mutated PY motif in ENaC to properly bind Nedd4-2. This leads to accumulation of active channels at the cell surface and increased Na+ (and fluid) absorption in the distal nephron, resulting in elevated blood volume and blood pressure. Small molecules/compounds that destabilize cell surface ENaC, or enhance Nedd4-2 activity in the kidney, could potentially serve to alleviate hypertension. PUBLICATION HISTORY : Republished from Current BioData's Targeted Proteins database (TPdb; http://www.targetedproteinsdb.com).
Figures
Similar articles
-
ENaC and its regulatory proteins as drug targets for blood pressure control.Curr Drug Targets. 2008 Aug;9(8):709-16. doi: 10.2174/138945008785132367. Curr Drug Targets. 2008. PMID: 18691017 Review.
-
Hrs controls sorting of the epithelial Na+ channel between endosomal degradation and recycling pathways.J Biol Chem. 2010 Oct 1;285(40):30523-30. doi: 10.1074/jbc.M110.150755. Epub 2010 Jul 30. J Biol Chem. 2010. PMID: 20675381 Free PMC article.
-
The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool.Traffic. 2007 Sep;8(9):1246-64. doi: 10.1111/j.1600-0854.2007.00602.x. Epub 2007 Jul 1. Traffic. 2007. PMID: 17605762
-
Hypoxia-induced inhibition of epithelial Na(+) channels in the lung. Role of Nedd4-2 and the ubiquitin-proteasome pathway.Am J Respir Cell Mol Biol. 2014 Mar;50(3):526-37. doi: 10.1165/rcmb.2012-0518OC. Am J Respir Cell Mol Biol. 2014. PMID: 24093724
-
Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination.Kidney Int. 2000 Mar;57(3):809-15. doi: 10.1046/j.1523-1755.2000.00919.x. Kidney Int. 2000. PMID: 10720933 Review.
Cited by
-
Ubiquitin Engineering for Interrogating the Ubiquitin-Proteasome System and Novel Therapeutic Strategies.Cells. 2023 Aug 21;12(16):2117. doi: 10.3390/cells12162117. Cells. 2023. PMID: 37626927 Free PMC article. Review.
-
The ubiquitin system, disease, and drug discovery.BMC Biochem. 2008 Oct 21;9 Suppl 1(Suppl 1):S7. doi: 10.1186/1471-2091-9-S1-S7. BMC Biochem. 2008. PMID: 19007437 Free PMC article. Review.
-
Ubiquitylation and the pathogenesis of hypertension.J Clin Invest. 2013 Feb;123(2):546-8. doi: 10.1172/JCI66882. Epub 2013 Jan 25. J Clin Invest. 2013. PMID: 23348731 Free PMC article.
-
Viruses go modular.J Biol Chem. 2020 Apr 3;295(14):4604-4616. doi: 10.1074/jbc.REV119.012414. Epub 2020 Feb 28. J Biol Chem. 2020. PMID: 32111739 Free PMC article. Review.
-
Genome-wide approaches to systematically identify substrates of the ubiquitin-proteasome pathway.Trends Biotechnol. 2010 Sep;28(9):461-7. doi: 10.1016/j.tibtech.2010.06.003. Epub 2010 Jul 14. Trends Biotechnol. 2010. PMID: 20637515 Free PMC article. Review.
References
-
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. - PubMed
-
- Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. - PubMed
-
- Rossier BC. The epithelial sodium channel: activation by membrane-bound serine proteases. Proc Am Thorac Soc. 2004;1:4–9. - PubMed
-
- Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC. Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol. 1994;127:1907–1921. - PMC - PubMed
-
- Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Renal Physiol. 2004;286:F669–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

