The UPS in diabetes and obesity
- PMID: 19007436
- PMCID: PMC2582800
- DOI: 10.1186/1471-2091-9-S1-S6
The UPS in diabetes and obesity
Abstract
Type 2 diabetes is caused by defects in both insulin signaling and insulin secretion. Though the role of the ubiquitin proteasome system (UPS) in the pathogenesis of type 2 diabetes remains largely unexplored, the few examples present in the literature are interesting and suggest targets for drug development. Studies indicate that insulin resistance can be induced by stimulating the degradation of important molecules in the insulin signaling pathway, in particular the insulin receptor substrate proteins IRS1, IRS2 and the kinase AKT1 (Akt). In addition, a defect in insulin secretion could occur due to UPS-mediated degradation of IRS2 in the beta-cells of the pancreas. The UPS also appears to be involved in regulating lipid synthesis in adipocytes and lipid production by the liver and could influence the development of obesity. Other possible mechanisms for inducing defects in insulin signaling and secretion remain to be explored, including the role of ubiquitylation in insulin receptor internalization and trafficking. PUBLICATION HISTORY : Republished from Current BioData's Targeted Proteins database (TPdb; http://www.targetedproteinsdb.com).
Figures
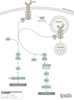
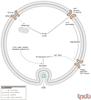
References
-
- Satoh S, Nishimura H, Clark AE, Kozka IJ, Vannucci SJ, Simpson IA, Quon MJ, Cushman SW, Holman GD. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J Biol Chem. 1993;268:17820–17829. - PubMed
-
- Buse JB, Polonsky KS, Burant CF. Type 2 Diabetes Mellitus. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editor. Williams Textbook of Endocrinology. 10. Philadelphia: Saunders; 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

