Dynamin 3 participates in the growth and development of megakaryocytes
- PMID: 19007685
- PMCID: PMC2728587
- DOI: 10.1016/j.exphem.2008.08.010
Dynamin 3 participates in the growth and development of megakaryocytes
Abstract
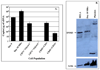
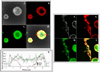
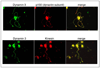
High-density oligonucleotide microarrays were used to compare gene expression profiles from uncultured CD34+/CD38lo cells and culture-derived megakaryocytes (MKs). As previously published, three replicate microarray data sets from three different sources of organ donor marrow were analyzed using the software program Rosetta Resolver. After setting a stringent p value of <or=0.001 with a fold change cutoff of three or more in expression level, dynamin 3 (DNM3) was identified to be differentially expressed during the course of MK development with a mean fold-change of 8.2+/-2.1 (mean+/-standard deviation). DNM3 is a member of a family of mechanochemical enzymes (DNM1, DNM2, and DNM3) known for their participation in membrane dynamics by hydrolyzing nucleotides to link cellular membranes to the actin cytoskeleton. Real-time quantitative polymerase chain reaction confirmed that DNM3 increased by 20.7-+/-3.4-fold (n=4, p=0.09) during megakaryocytopoiesis and Western blot analysis showed that DNM3 protein was expressed in human MKs. Confocal microscopy revealed that DNM3 was distributed diffusely throughout the cytoplasm of MKs with a punctate appearance in proplatelet processes. Immunogold electron microscopy also showed that DNM3 is widely distributed in the cytoplasm of MKs, with no apparent localization to specific organelles. The open reading frame of DNM3 was cloned from culture-derived human MKs and determined to be 100% identical to the protein encoded by the DNM3 transcript variant ENST00000367731 published in the Ensemble database. Overexpression of DNM3 in umbilical cord blood CD34+ cells resulted in an increase in total nucleated cells, an amplification of total colony-forming cells and colony-forming unit-megakaryocytes, and a concomitant increase in the expression of nuclear factor erythroid 2 (NF-E2) and beta-tubulin. Together these findings provide the first evidence that a member of the dynamin family of mechanochemical enzymes is present in human MKs and indicate that DNM3 is an excellent candidate for playing an important role in mediating cytoskeleton and membrane changes that occur during MK/platelet development.
Figures






References
-
- Orth JD, McNiven MA. Dynamin at the actin-membrane interface [review] Curr Opin Cell Biol. 2003;15:31–39. - PubMed
-
- Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59:421–432. - PubMed
-
- Krutchen AE, McNiven MA. Dynamin as a mover and pincher during cell migration and invasion. J Cell Sci. 2006;119:1683–1690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

