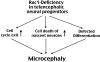
Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly
- PMID: 19007770
- PMCID: PMC2653853
- DOI: 10.1016/j.ydbio.2008.10.023
Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly
Abstract
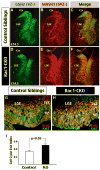
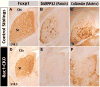
The Rho family of small GTPases has been implicated in many neurological disorders including mental retardation, but whether they are involved in primary microcephaly (microcephalia vera) is unknown. Here, we examine the role of Rac1 in mammalian neural progenitors and forebrain development by a conditional gene-targeting strategy using the Foxg1-Cre line to delete floxed-Rac1 alleles in the telencephalic ventricular zone (VZ) of mouse embryos. We found that Rac1 deletion in the telencephalic VZ progenitors resulted in reduced sizes of both the striatum and cerebral cortex. Analyses further indicated that this abnormality was caused by accelerated cell-cycle exit and increased apoptosis during early corticogenesis (approximately E14.5), leading to a decrease of the neural progenitor pool in mid-to-late telencephalic development (E16.5 to E18.5). Moreover, the formation of patch-matrix compartments in the striatum was impaired by Rac1-deficiency. Together, these results suggest that Rac1 regulates self-renewal, survival, and differentiation of telencephalic neural progenitors, and that dysfunctions of Rac1 may lead to primary microcephaly.
Figures



References
-
- Abuelo D. Microcephaly syndromes. Semin Pediatr Neurol. 2007;14:118–127. - PubMed
-
- Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–935. - PubMed
-
- Bond J, Scott S, Hampshire DJ, Springell K, Corry P, Abramowicz MJ, Mochida GH, Hennekam RC, Maher ER, Fryns JP, Alswaid A, Jafri H, Rashid Y, Mubaidin A, Walsh CA, Roberts E, Woods CG. Protein-truncating mutations in ASPM cause variable reduction in brain size. Am J Hum Genet. 2003;73:1170–7. - PMC - PubMed
-
- de la Rosa EJ, de Pablo F. Cell death in early neural development: Beyond the neurotrophic theory. Trends Neurosci. 2000;23:454–58. - PubMed
-
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–37. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

