Essential role of PACT-mediated PKR activation in tunicamycin-induced apoptosis
- PMID: 19007793
- PMCID: PMC4026198
- DOI: 10.1016/j.jmb.2008.10.068
Essential role of PACT-mediated PKR activation in tunicamycin-induced apoptosis
Abstract
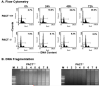
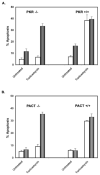
Cellular stresses such as disruption of calcium homeostasis, inhibition of protein glycosylation, and reduction of disulfide bonds result in accumulation of misfolded proteins in the endoplasmic reticulum (ER) and lead to cell death by apoptosis. Tunicamycin, which is an inhibitor of protein glycosylation, induces ER stress and apoptosis. In this study, we examined the involvement of double-stranded RNA (dsRNA)-activated protein kinase (PKR) and its protein activator PACT in tunicamycin-induced apoptosis. We demonstrate for the first time that PACT is phosphorylated in response to tunicamycin and is responsible for PKR activation by direct interaction. Furthermore, PACT-induced PKR activation is essential for tunicamycin-induced apoptosis, since PACT as well as PKR null cells are markedly resistant to tunicamycin and show defective eIF2alpha phosphorylation and C/EBP homologous protein (CHOP, also known as GADD153) induction especially at low concentrations of tunicamycin. Reconstitution of PKR and PACT expression in the null cells renders them sensitive to tunicamycin, thus demonstrating that PACT-induced PKR activation plays an essential function in induction of apoptosis.
Figures

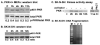




Similar articles
-
Inhibition of PKR protects against tunicamycin-induced apoptosis in neuroblastoma cells.Gene. 2014 Feb 15;536(1):90-6. doi: 10.1016/j.gene.2013.11.074. Epub 2013 Dec 14. Gene. 2014. PMID: 24334130
-
PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR.J Biol Chem. 2000 Dec 1;275(48):37993-8. doi: 10.1074/jbc.M004762200. J Biol Chem. 2000. PMID: 10988289
-
An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer's disease.EMBO J. 2004 Feb 25;23(4):959-68. doi: 10.1038/sj.emboj.7600049. Epub 2004 Feb 5. EMBO J. 2004. PMID: 14765129 Free PMC article.
-
PACT and PKR: turning on NF-kappa B in the absence of virus.Sci STKE. 2001 Jul 3;2001(89):re1. doi: 10.1126/stke.2001.89.re1. Sci STKE. 2001. PMID: 11752660 Review.
-
A tale of two proteins: PACT and PKR and their roles in inflammation.FEBS J. 2021 Nov;288(22):6365-6391. doi: 10.1111/febs.15691. Epub 2021 Jan 15. FEBS J. 2021. PMID: 33387379 Free PMC article. Review.
Cited by
-
Altered activation of protein kinase PKR and enhanced apoptosis in dystonia cells carrying a mutation in PKR activator protein PACT.J Biol Chem. 2015 Sep 11;290(37):22543-57. doi: 10.1074/jbc.M115.669408. Epub 2015 Jul 31. J Biol Chem. 2015. PMID: 26231208 Free PMC article.
-
Regulation of PKR activation and apoptosis during oxidative stress by TRBP phosphorylation.Int J Biochem Cell Biol. 2021 Aug;137:106030. doi: 10.1016/j.biocel.2021.106030. Epub 2021 Jun 24. Int J Biochem Cell Biol. 2021. PMID: 34174402 Free PMC article.
-
Immunogenic cell death triggered by impaired deubiquitination in multiple myeloma relies on dysregulated type I interferon signaling.Front Immunol. 2023 Mar 2;14:982720. doi: 10.3389/fimmu.2023.982720. eCollection 2023. Front Immunol. 2023. PMID: 36936919 Free PMC article.
-
mRNA decapping enzyme 1a (Dcp1a)-induced translational arrest through protein kinase R (PKR) activation requires the N-terminal enabled vasodilator-stimulated protein homology 1 (EVH1) domain.J Biol Chem. 2014 Feb 14;289(7):3936-49. doi: 10.1074/jbc.M113.518191. Epub 2013 Dec 31. J Biol Chem. 2014. PMID: 24382890 Free PMC article.
-
Luteolin protects DYT-PRKRA cells from apoptosis by suppressing PKR activation.Front Pharmacol. 2023 Feb 15;14:1118725. doi: 10.3389/fphar.2023.1118725. eCollection 2023. Front Pharmacol. 2023. PMID: 36874028 Free PMC article.
References
-
- Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. - PubMed
-
- Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. - PubMed
-
- Hovanessian AG, Galabru J. The double-stranded RNA-dependent protein kinase is also activated by heparin. Eur. J. Biochem. 1987;167:467–473. - PubMed
-
- Sadler AJ, Williams BR. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 2007;316:253–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

