Rapid, semi-automated, and inexpensive radioimmunoassay of cAMP: application in GPCR-mediated adenylate cyclase assays
- PMID: 19007813
- PMCID: PMC2717699
- DOI: 10.1016/j.jneumeth.2008.10.016
Rapid, semi-automated, and inexpensive radioimmunoassay of cAMP: application in GPCR-mediated adenylate cyclase assays
Abstract
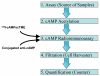
Cyclic AMP (cAMP) is an important signal transduction second messenger that is commonly used as a functional mirror on the actions of G protein-coupled receptors that can activate or inhibit adenylate cyclases. A radioimmunoassay for cAMP with femtomole sensitivity was first reported by Steiner more than 30 years ago, and there have been several subsequent modifications that have improved this assay in various ways. Here we describe additional improvement to existing methods that markedly improve speed and reduce cost without sacrificing sensitivity, and is also adaptable to analysis of cGMP. The primary antibody is coupled directly to magnetic beads that are then separated from unbound marker using filtration on microplates. This eliminates the need for a secondary antibody, and markedly increases throughput. In addition, we report a simple, reproducible, and inexpensive method to make the radiomarker used for this assay. Although still requiring the use of radioactivity, the resulting method retains a high degree of accuracy and precision, and is suitable for low-cost high throughput screening. Use of aspects of this method can also improve throughput in other radioimmunoassays.
Figures
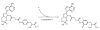




Similar articles
-
Newer developments in the determination of cyclic AMP and other cyclic nucleotides, adenylate cyclase, and phosphodiesterase.Methods Biochem Anal. 1974;22:95-121. doi: 10.1002/9780470110423.ch2. Methods Biochem Anal. 1974. PMID: 4373637 No abstract available.
-
Methods related to cyclic AMP and adenylate cyclase.Prog Med Chem. 1975;12:293-331. doi: 10.1016/s0079-6468(08)70179-6. Prog Med Chem. 1975. PMID: 7803 Review. No abstract available.
-
Improvements in the automated radioimmunoassay for cAMP or cGMP.Methods Enzymol. 1988;159:45-50. doi: 10.1016/0076-6879(88)59006-7. Methods Enzymol. 1988. PMID: 2842610 No abstract available.
-
Comparative analysis between cyclic GMP and cyclic AMP signalling in human sperm.Mol Hum Reprod. 2004 Jul;10(7):543-52. doi: 10.1093/molehr/gah065. Epub 2004 Apr 30. Mol Hum Reprod. 2004. PMID: 15121877
-
cAMP signaling in Mycobacterium tuberculosis.Indian J Exp Biol. 2009 Jun;47(6):393-400. Indian J Exp Biol. 2009. PMID: 19634702 Review.
Cited by
-
Establishing a sensitive fluorescence-based quantification method for cyclic nucleotides.BMC Biotechnol. 2020 Aug 27;20(1):47. doi: 10.1186/s12896-020-00633-y. BMC Biotechnol. 2020. PMID: 32854679 Free PMC article.
-
Downregulated INHBB in endometrial tissue of recurrent implantation failure patients impeded decidualization through the ADCY1/cAMP signalling pathway.J Assist Reprod Genet. 2023 May;40(5):1135-1146. doi: 10.1007/s10815-023-02762-7. Epub 2023 Mar 13. J Assist Reprod Genet. 2023. PMID: 36913138 Free PMC article.
-
Receptor conformations involved in dopamine D(2L) receptor functional selectivity induced by selected transmembrane-5 serine mutations.Mol Pharmacol. 2012 Jun;81(6):820-31. doi: 10.1124/mol.111.075457. Epub 2012 Mar 13. Mol Pharmacol. 2012. PMID: 22416052 Free PMC article.
-
Integration of signal transduction pathways in sensory epithelial enteroendocrine cells.Mol Biol Cell. 2025 Jun 1;36(6):re3. doi: 10.1091/mbc.E24-03-0143. Mol Biol Cell. 2025. PMID: 40408598 Review.
-
SKF-83959 is not a highly-biased functionally selective D1 dopamine receptor ligand with activity at phospholipase C.Neuropharmacology. 2014 Nov;86:145-54. doi: 10.1016/j.neuropharm.2014.05.042. Epub 2014 Jun 12. Neuropharmacology. 2014. PMID: 24929112 Free PMC article.
References
-
- Antoni FA. Molecular diversity of cyclic AMP signalling. Front Neuroendocrinol. 2000;21:103–132. - PubMed
-
- Brewster WK, Nichols DE, Riggs RM, Mottola DM, Lovenberg TW, Lewis MH, Mailman RB. trans-10,11-dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine: a highly potent selective dopamine D1 full agonist. J.Med.Chem. 1990;33:1756–1764. - PubMed
-
- Brooker G, Terasaki WL, Price MG. Gammaflow: a completely automated radioimmunoassay system. Science. 1976;194:270–276. - PubMed
-
- Brown BL, Ekins RP, Albano JD. Saturation assay for cyclic AMP using endogenous binding protein. Adv.Cyclic.Nucleotide.Res. 1972;2:25–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

