A novel DPP6 isoform (DPP6-E) can account for differences between neuronal and reconstituted A-type K(+) channels
- PMID: 19007856
- PMCID: PMC2820293
- DOI: 10.1016/j.neulet.2008.10.098
A novel DPP6 isoform (DPP6-E) can account for differences between neuronal and reconstituted A-type K(+) channels
Abstract
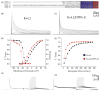
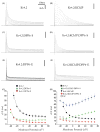
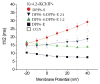
The channels mediating most of the somatodendritic A-type K(+) current in neurons are thought to be ternary complexes of Kv4 pore-forming subunits and two types of auxiliary subunits, the K(+) channel interacting proteins (KChIPs) and dipeptidyl-peptidase-like (DPPL) proteins. The channels expressed in heterologous expression systems by mixtures of Kv4.2, KChIP1 and DPP6-S resemble in many properties the A-type current in hippocampal CA1 pyramidal neurons and cerebellar granule cells, neurons with prominent A-type K(+) currents. However, the native currents have faster kinetics. Moreover, the A-type currents in neurons in intermediary layers of the superior colliculus have even faster inactivating rates. We have characterized a new DPP6 spliced isoform, DPP6-E, that produces in heterologous cells ternary Kv4 channels with very fast kinetics. DPP6-E is selectively expressed in a few neuronal populations in brain including cerebellar granule neurons, hippocampal pyramidal cells and neurons in intermediary layers of the superior colliculus. The effects of DPP6-E explain past discrepancies between reconstituted and native Kv4 channels in some neurons, and contributes to the diversity of A-type K(+) currents in neurons.
Figures

Similar articles
-
Neuronal Roles of the Multifunctional Protein Dipeptidyl Peptidase-like 6 (DPP6).Int J Mol Sci. 2022 Aug 16;23(16):9184. doi: 10.3390/ijms23169184. Int J Mol Sci. 2022. PMID: 36012450 Free PMC article. Review.
-
I SA channel complexes include four subunits each of DPP6 and Kv4.2.J Biol Chem. 2008 May 30;283(22):15072-7. doi: 10.1074/jbc.M706964200. Epub 2008 Mar 25. J Biol Chem. 2008. PMID: 18364354 Free PMC article.
-
Coexpression of auxiliary subunits KChIP and DPPL in potassium channel Kv4-positive nociceptors and pain-modulating spinal interneurons.J Comp Neurol. 2016 Mar 1;524(4):846-73. doi: 10.1002/cne.23876. Epub 2015 Aug 18. J Comp Neurol. 2016. PMID: 26239200
-
Effects of MiRP1 and DPP6 beta-subunits on the blockade induced by flecainide of Kv4.3/KChIP2 channels.Br J Pharmacol. 2008 Jun;154(4):774-86. doi: 10.1038/bjp.2008.134. Epub 2008 Apr 21. Br J Pharmacol. 2008. PMID: 18536731 Free PMC article.
-
Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons.J Physiol. 2008 Dec 1;586(23):5609-23. doi: 10.1113/jphysiol.2008.161620. Epub 2008 Oct 9. J Physiol. 2008. PMID: 18845608 Free PMC article. Review.
Cited by
-
Daily electrical activity in the master circadian clock of a diurnal mammal.Elife. 2021 Nov 30;10:e68179. doi: 10.7554/eLife.68179. Elife. 2021. PMID: 34845984 Free PMC article.
-
Neuronal Roles of the Multifunctional Protein Dipeptidyl Peptidase-like 6 (DPP6).Int J Mol Sci. 2022 Aug 16;23(16):9184. doi: 10.3390/ijms23169184. Int J Mol Sci. 2022. PMID: 36012450 Free PMC article. Review.
-
S-glutathionylation of an auxiliary subunit confers redox sensitivity to Kv4 channel inactivation.PLoS One. 2014 Mar 27;9(3):e93315. doi: 10.1371/journal.pone.0093315. eCollection 2014. PLoS One. 2014. PMID: 24675763 Free PMC article.
-
The prion protein modulates A-type K+ currents mediated by Kv4.2 complexes through dipeptidyl aminopeptidase-like protein 6.J Biol Chem. 2013 Dec 27;288(52):37241-55. doi: 10.1074/jbc.M113.488650. Epub 2013 Nov 13. J Biol Chem. 2013. PMID: 24225951 Free PMC article.
-
Dipeptidyl peptidase-like protein 6 is required for normal electrophysiological properties of cerebellar granule cells.J Neurosci. 2010 Jun 23;30(25):8551-65. doi: 10.1523/JNEUROSCI.5489-09.2010. J Neurosci. 2010. PMID: 20573902 Free PMC article.
References
-
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. - PubMed
-
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

