Mutations in the Chinese hamster ovary cell GART gene of de novo purine synthesis
- PMID: 19007868
- PMCID: PMC2643067
- DOI: 10.1016/j.gene.2008.10.007
Mutations in the Chinese hamster ovary cell GART gene of de novo purine synthesis
Abstract
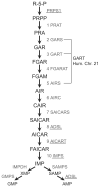
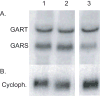
Mutations in several steps of de novo purine synthesis lead to human inborn errors of metabolism often characterized by mental retardation, hypotonia, sensorineural hearing loss, optic atrophy, and other features. In animals, the phosphoribosylglycinamide transformylase (GART) gene encodes a trifunctional protein carrying out 3 steps of de novo purine synthesis, phosphoribosylglycinamide synthase (GARS), phosphoribosylglycinamide transformylase (also abbreviated as GART), and phosphoribosylaminoimidazole synthetase (AIRS) and a smaller protein that contains only the GARS domain of GART as a functional protein. The GART gene is located on human chromosome 21 and is aberrantly regulated and overexpressed in individuals with Down syndrome (DS), and may be involved in the phenotype of DS. The GART activity of GART requires 10-formyltetrahydrofolate and has been a target for anti-cancer drugs. Thus, a considerable amount of information is available about GART, while less is known about the GARS and AIRS domains. Here we demonstrate that the amino acid residue glu75 is essential for the activity of the GARS enzyme and that the gly684 residue is essential for the activity of the AIRS enzyme by analysis of mutations in the Chinese hamster ovary (CHO-K1) cell that require purines for growth. We report the effects of these mutations on mRNA and protein content for GART and GARS. Further, we discuss the likely mechanisms by which mutations inactivating the GART protein might arise in CHO-K1 cells.
Figures
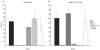

References
-
- Aimi J, Qiu H, Williams J, Dixon JE. De novo purine nucleotide biosynthesis: cloning of human and avian cDNAs encoding the trifunctional glycinamide ribonucleotide synthetase-aminoimidazole ribonucleotide synthetase-glycinamide ribonucleotide transformylase by functional complementation in E. coli. Nuc Acid Res. 1990;18:6665–6672. - PMC - PubMed
-
- An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–6. - PubMed
-
- Ausubel FM. Current Protocols in Molecular Biology. J Wiley; Brooklyn, NY: 1987.
-
- Barnes TS, Bleskan JH, Hart IM, Walton KM, Barton JW, Patterson D. Purification of, generation of monoclonal antibodies to, and mapping of phosphoribosyl N-formylglycinamide amidotransferase. Biochemistry. 1994;33:1850–1860. - PubMed
-
- Becker MA, Nosal JM, Switzer RL, Smith PR, Palella TD, Roessler BJ. Point mutations in PRPS1, the gene encoding the PRPP synthetase (PRS) 1 isoform, underlie X-linked PRS superactivity associated with purine nucleotide inhibitor-resistance. Adv Exp Med Biol. 1994;370:707–710. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

