Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity
- PMID: 19007880
- PMCID: PMC2630396
- DOI: 10.1016/j.cellsig.2008.10.014
Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity
Abstract
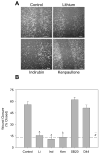
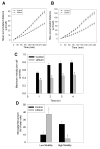
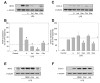
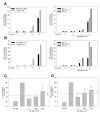
Microglia play a prominent role in the brain's inflammatory response to injury or infection by migrating to affected locations, secreting inflammatory molecules, and phagocytosing damaged tissue. However, because severe or chronic neuroinflammation exacerbates many neurological conditions, controlling microglia actions may provide therapeutic benefits in a diverse array of diseases. Since glycogen synthase kinase-3 (GSK3) promotes inflammatory responses in peripheral immune cells, we investigated if inhibitors of GSK3 attenuated microglia responses to inflammatory stimuli. Treatment of BV-2 microglia with GSK3 inhibitors greatly reduced the migration of microglia in both a scratch assay and in a transwell migration assay. Treatment of BV-2 microglia with lipopolysaccharide (LPS) stimulated the production of interleukin-6 and increased the expression of inducible nitric oxide synthase (iNOS) and NO production. Each of these microglia responses to inflammatory stimulation were greatly attenuated by GSK3 inhibitors. However, GSK3 inhibitors did not cause a general impairment of microglia functions, as the LPS-induced stimulated expression of cyclooxygenase-2 was unaltered. Regulation of microglia functions were also evident in cultured mouse hippocampal slices where GSK3 inhibitors reduced cytokine production and microglial migration, and provided protection from inflammation-induced neuronal toxicity. These findings demonstrate that GSK3 promotes microglial responses to inflammation and that the utilization of GSK3 inhibitors provides a means to limit the inflammatory actions of microglia.
Figures




References
-
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. Nat Neurosci. 2005;8:752. - PubMed
-
- Nimmerjahn A, Kirchhoff F, Helmchen F. Science. 2005;308:1314. - PubMed
-
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Brain Res Rev. 2005;48:196. - PubMed
-
- Hanisch UK, Kettenmann H. Nat Neurosci. 2007;10:1387. - PubMed
-
- Kim SU, de Vellis J. J Neurosci Res. 2005;81:302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

