Products and substrate/template usage of vaccinia virus DNA primase
- PMID: 19007959
- PMCID: PMC2630392
- DOI: 10.1016/j.virol.2008.10.008
Products and substrate/template usage of vaccinia virus DNA primase
Abstract
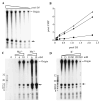
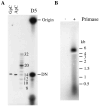
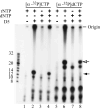
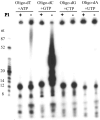
Vaccinia virus encodes a 90-kDa protein conserved in all poxviruses, with DNA primase and nucleoside triphosphatase activities. DNA primase products, synthesized with a single stranded varphiX174 DNA template, were resolved as dinucleotides and long RNAs on denaturing polyacrylamide and agarose gels. Following phosphatase treatment, the dinucleotides GpC and ApC in a 4:1 ratio were identified by nearest neighbor analysis in which (32)P was transferred from [alpha-(32)P]CTP to initiating purine nucleotides. Differences in the nucleotide binding sites for initiation and elongation were suggested by the absence of CpC and UpC dinucleotides as well as the inability of deoxynucleotides to mediate primer synthesis despite their incorporation into mixed RNA/DNA primers. Strong primase activity was detected with an oligo(dC) template. However, there was only weak activity with an oligo(dT) template and none with oligo(dA) or oligo(dG). The absence of stringent template specificity is consistent with a role for the enzyme in priming DNA synthesis at the replication fork.
Figures

References
-
- Baroudy BM, Venkatesan S, Moss B. Incompletely base-paired flipflop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell. 1982a;28:315–324. - PubMed
-
- Baroudy BM, Venkatesan S, Moss B. Structure and replication of vaccinia virus telomeres. Cold Spring Harbor Symp Quant Biol. 1982b;47:723–729. - PubMed
-
- Bocquier AA, Liu L, Cann IK, Komori K, Kohda D, Ishino Y. Archaeal primase: bridging the gap between RNA and DNA polymerases. Curr Biol. 2001;11:452–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

