CXCR4 mediates the proliferation of glioblastoma progenitor cells
- PMID: 19008040
- PMCID: PMC2628453
- DOI: 10.1016/j.canlet.2008.09.034
CXCR4 mediates the proliferation of glioblastoma progenitor cells
Abstract
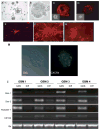
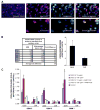
Increasing evidence points to a fundamental role for cancer stem cells (CSC) in the initiation and propagation of many tumors. As such, in the context of glioblastoma multiforme (GBM), the development of treatment strategies specifically targeted towards CSC-like populations may hold significant therapeutic promise. To this end, we now report that the cell surface chemokine receptor, CXCR4, a known mediator of cancer cell proliferation and invasion, is overexpressed in primary glioblastoma progenitor cells versus corresponding differentiated tumor cells. Furthermore, administration of CXCL12, the only known ligand for CXCR4, stimulates a specific and significant proliferative response in progenitors but not differentiated tumor cells. Taken together, these results implicate an important role for the CXCR4 signaling mechanism in glioma CSC biology and point to the therapeutic potential of targeting this pathway in patients with GBM.
Conflict of interest statement
Figures

Similar articles
-
Inhibition of CXCL12/CXCR4 autocrine/paracrine loop reduces viability of human glioblastoma stem-like cells affecting self-renewal activity.Toxicology. 2013 Dec 15;314(2-3):209-20. doi: 10.1016/j.tox.2013.10.003. Epub 2013 Oct 21. Toxicology. 2013. PMID: 24157575
-
CXCL12 mediates trophic interactions between endothelial and tumor cells in glioblastoma.PLoS One. 2012;7(3):e33005. doi: 10.1371/journal.pone.0033005. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22427929 Free PMC article.
-
CXCL12/CXCR4 promotes motility and proliferation of glioma cells.Cancer Biol Ther. 2010 Jan;9(1):56-65. doi: 10.4161/cbt.9.1.10342. Epub 2010 Jan 17. Cancer Biol Ther. 2010. PMID: 19923906
-
CXCL12/CXCR4 signaling in malignant brain tumors: a potential pharmacological therapeutic target.Brain Tumor Pathol. 2011 Apr;28(2):89-97. doi: 10.1007/s10014-010-0013-1. Epub 2011 Jan 6. Brain Tumor Pathol. 2011. PMID: 21210239 Review.
-
The role of the CXCR4 cell surface chemokine receptor in glioma biology.J Neurooncol. 2013 Jun;113(2):153-62. doi: 10.1007/s11060-013-1108-4. Epub 2013 Mar 14. J Neurooncol. 2013. PMID: 23494875 Review.
Cited by
-
Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth.Cell. 2013 Mar 28;153(1):139-52. doi: 10.1016/j.cell.2013.02.021. Cell. 2013. PMID: 23540695 Free PMC article.
-
(68)Ga-Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 Expression in Glioblastoma.Theranostics. 2016 Jan 25;6(3):428-34. doi: 10.7150/thno.13986. eCollection 2016. Theranostics. 2016. PMID: 26909116 Free PMC article.
-
The Role of Cytokines and Chemokines in Shaping the Immune Microenvironment of Glioblastoma: Implications for Immunotherapy.Cells. 2021 Mar 9;10(3):607. doi: 10.3390/cells10030607. Cells. 2021. PMID: 33803414 Free PMC article. Review.
-
Inhibition of stromal CXCR4 impairs development of lung metastases.Cancer Immunol Immunother. 2012 Oct;61(10):1713-20. doi: 10.1007/s00262-012-1223-7. Epub 2012 Mar 8. Cancer Immunol Immunother. 2012. PMID: 22399057 Free PMC article.
-
Cryptotanshinone targets tumor-initiating cells through down-regulation of stemness genes expression.Oncol Lett. 2016 Jun;11(6):3803-3812. doi: 10.3892/ol.2016.4444. Epub 2016 Apr 15. Oncol Lett. 2016. PMID: 27313698 Free PMC article.
References
-
- Aghi M, Cohen KS, et al. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. - PubMed
-
- Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. - PubMed
-
- Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. - PubMed
-
- Bao S, Wu Q, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. - PubMed
-
- Bao S, Wu Q, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

