Sprouty 2 regulates DNA damage-induced apoptosis in Ras-transformed human fibroblasts
- PMID: 19008219
- PMCID: PMC2613613
- DOI: 10.1074/jbc.M808045200
Sprouty 2 regulates DNA damage-induced apoptosis in Ras-transformed human fibroblasts
Abstract
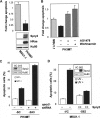
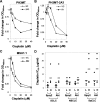
We have reported that expression of Sprouty 2 (Spry2) is necessary for tumor formation by HRas(V12)-transformed fibroblasts. We now report on the role of Spry2 in the inhibition of UV(254 nm) radiation-induced apoptosis in HRas(V12)-transformed human fibroblasts. Silencing Spry2 in this context resulted in increased apoptosis, associated with decreased Akt activation and decreased phosphorylation of HDM2 at Ser-166, which has been shown to stabilize HDM2. As a consequence, when cells with silenced Spry2 were UV-irradiated, they exhibited diminished levels of HDM2 and elevated levels of p53. In agreement with these findings, overexpression of Spry2 in the parental non-transformed fibroblasts led to increased Akt activation and to the stabilization of HDM2. It also led to diminished expression of p53 and decreased apoptosis following UV irradiation. Silencing Spry2 in HRas-transformed cells decreased Rac1 activation, but independent expression of Spry2 in the non-transformed parental cells had no effect on Rac1, suggesting a specific involvement in the activation of Rac1 by Ras. Silencing Spry2 in HRas(V12)-transformed cells resulted in diminished interaction between HRas and Tiam1, a Rac1-specific nucleotide exchange factor. Expression of constitutively active Rac1 in cells with silenced Spry2 partly reversed the effect of Spry2 down-regulation. Furthermore, loss of Spry2 expression in HRas(V12)-transformed cells augmented the cytotoxicity of the DNA-damaging, chemotherapeutic agent cisplatin, a process that was also reversed by active Rac1. Together, these data show that Spry2 inhibits apoptosis in response to DNA damage by regulating Akt, HDM2, and p53, by a process mediated partly by Rac1.
Figures



Similar articles
-
Evidence that sprouty 2 is necessary for sarcoma formation by H-Ras oncogene-transformed human fibroblasts.J Biol Chem. 2008 Jan 25;283(4):2002-9. doi: 10.1074/jbc.M709046200. Epub 2007 Nov 28. J Biol Chem. 2008. PMID: 18048363
-
Rac1 and Cdc42 are regulators of HRasV12-transformation and angiogenic factors in human fibroblasts.BMC Cancer. 2010 Jan 12;10:13. doi: 10.1186/1471-2407-10-13. BMC Cancer. 2010. PMID: 20067638 Free PMC article.
-
Sprouty regulates cell migration by inhibiting the activation of Rac1 GTPase.Biochem Biophys Res Commun. 2004 Oct 8;323(1):98-103. doi: 10.1016/j.bbrc.2004.08.070. Biochem Biophys Res Commun. 2004. PMID: 15351707
-
Oncogenic H-Ras enhances DNA repair through the Ras/phosphatidylinositol 3-kinase/Rac1 pathway in NIH3T3 cells. Evidence for association with reactive oxygen species.J Biol Chem. 2002 May 31;277(22):19358-66. doi: 10.1074/jbc.M200933200. Epub 2002 Mar 7. J Biol Chem. 2002. PMID: 11884408
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
Sprouty1, a new target of the angiostatic agent 16K prolactin, negatively regulates angiogenesis.Mol Cancer. 2010 Sep 2;9:231. doi: 10.1186/1476-4598-9-231. Mol Cancer. 2010. PMID: 20813052 Free PMC article.
-
Intermolecular interactions of Sprouty proteins and their implications in development and disease.Mol Pharmacol. 2009 Oct;76(4):679-91. doi: 10.1124/mol.109.055848. Epub 2009 Jul 1. Mol Pharmacol. 2009. PMID: 19570949 Free PMC article. Review.
-
Spry1 as a novel regulator of erythropoiesis, EPO/EPOR target, and suppressor of JAK2.Blood. 2012 Jun 7;119(23):5522-31. doi: 10.1182/blood-2011-11-392571. Epub 2012 Apr 16. Blood. 2012. PMID: 22508938 Free PMC article.
-
The enhancement of Raf-1 kinase activity by knockdown of Spry2 is associated with high sensitivity to paclitaxel in v-Ha-ras-transformed NIH 3T3 fibroblasts.Mol Cell Biochem. 2009 Dec;332(1-2):189-97. doi: 10.1007/s11010-009-0191-5. Epub 2009 Jul 9. Mol Cell Biochem. 2009. PMID: 19588231
-
Systematic analysis of copy number variants of a large cohort of orofacial cleft patients identifies candidate genes for orofacial clefts.Hum Genet. 2016 Jan;135(1):41-59. doi: 10.1007/s00439-015-1606-x. Epub 2015 Nov 11. Hum Genet. 2016. PMID: 26561393 Free PMC article.
References
-
- Downward, J. (2003) Nat. Rev. Cancer 3 11–22 - PubMed
-
- Messina, S., Leonetti, C., De Gregorio, G., Affatigato, V., Ragona, G., Frati, L., Zupi, G., Santoni, A., and Porcellini, A. (2004) Biochem. Biophys. Res. Commun. 320 493–500 - PubMed
-
- Ries, S., Biederer, C., Woods, D., Shifman, O., Shirasawa, S., Sasazuki, T., McMahon, M., Oren, M., and McCormick, F. (2000) Cell 103 321–330 - PubMed
-
- Campbell, S. L., Khosravi-Far, R., Rossman, K. L., Clark, G. J., and Der, C. J. (1998) Oncogene 17 1395–1413 - PubMed
-
- Datta, S. R., Brunet, A., and Greenberg, M. E. (1999) Genes Dev. 13 2905–2927 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

