Proper restoration of excitation-contraction coupling in the dihydropyridine receptor beta1-null zebrafish relaxed is an exclusive function of the beta1a subunit
- PMID: 19008220
- PMCID: PMC2613631
- DOI: 10.1074/jbc.M807767200
Proper restoration of excitation-contraction coupling in the dihydropyridine receptor beta1-null zebrafish relaxed is an exclusive function of the beta1a subunit
Abstract
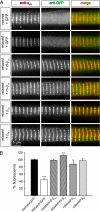
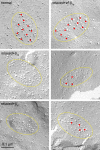
The paralyzed zebrafish strain relaxed carries a null mutation for the skeletal muscle dihydropyridine receptor (DHPR) beta(1a) subunit. Lack of beta(1a) results in (i) reduced membrane expression of the pore forming DHPR alpha(1S) subunit, (ii) elimination of alpha(1S) charge movement, and (iii) impediment of arrangement of the DHPRs in groups of four (tetrads) opposing the ryanodine receptor (RyR1), a structural prerequisite for skeletal muscle-type excitation-contraction (EC) coupling. In this study we used relaxed larvae and isolated myotubes as expression systems to discriminate specific functions of beta(1a) from rather general functions of beta isoforms. Zebrafish and mammalian beta(1a) subunits quantitatively restored alpha(1S) triad targeting and charge movement as well as intracellular Ca(2+) release, allowed arrangement of DHPRs in tetrads, and most strikingly recovered a fully motile phenotype in relaxed larvae. Interestingly, the cardiac/neuronal beta(2a) as the phylogenetically closest, and the ancestral housefly beta(M) as the most distant isoform to beta(1a) also completely recovered alpha(1S) triad expression and charge movement. However, both revealed drastically impaired intracellular Ca(2+) transients and very limited tetrad formation compared with beta(1a). Consequently, larval motility was either only partially restored (beta(2a)-injected larvae) or not restored at all (beta(M)). Thus, our results indicate that triad expression and facilitation of 1,4-dihydropyridine receptor (DHPR) charge movement are common features of all tested beta subunits, whereas the efficient arrangement of DHPRs in tetrads and thus intact DHPR-RyR1 coupling is only promoted by the beta(1a) isoform. Consequently, we postulate a model that presents beta(1a) as an allosteric modifier of alpha(1S) conformation enabling skeletal muscle-type EC coupling.
Figures
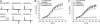

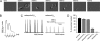

Similar articles
-
The distal C terminus of the dihydropyridine receptor β1a subunit is essential for tetrad formation in skeletal muscle.Proc Natl Acad Sci U S A. 2022 May 10;119(19):e2201136119. doi: 10.1073/pnas.2201136119. Epub 2022 May 4. Proc Natl Acad Sci U S A. 2022. PMID: 35507876 Free PMC article.
-
The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle.Proc Natl Acad Sci U S A. 2005 Nov 22;102(47):17219-24. doi: 10.1073/pnas.0508710102. Epub 2005 Nov 14. Proc Natl Acad Sci U S A. 2005. PMID: 16286639 Free PMC article.
-
Domain cooperativity in the β1a subunit is essential for dihydropyridine receptor voltage sensing in skeletal muscle.Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7488-93. doi: 10.1073/pnas.1301087110. Epub 2013 Apr 15. Proc Natl Acad Sci U S A. 2013. PMID: 23589859 Free PMC article.
-
Functional equivalence of dihydropyridine receptor alpha1S and beta1a subunits in triggering excitation-contraction coupling in skeletal muscle.Biol Res. 2004;37(4):565-75. doi: 10.4067/s0716-97602004000400010. Biol Res. 2004. PMID: 15709683 Review.
-
The role of auxiliary dihydropyridine receptor subunits in muscle.J Muscle Res Cell Motil. 2005;26(1):1-6. doi: 10.1007/s10974-005-9000-2. Epub 2005 Oct 14. J Muscle Res Cell Motil. 2005. PMID: 16088377 Review.
Cited by
-
Rem uncouples excitation-contraction coupling in adult skeletal muscle fibers.J Gen Physiol. 2015 Jul;146(1):97-108. doi: 10.1085/jgp.201411314. Epub 2015 Jun 15. J Gen Physiol. 2015. PMID: 26078055 Free PMC article.
-
Distribution of voltage gated calcium channel β subunits in the mouse retina.Brain Res. 2011 Sep 15;1412:1-8. doi: 10.1016/j.brainres.2011.07.033. Epub 2011 Jul 23. Brain Res. 2011. PMID: 21831364 Free PMC article.
-
NO-sGC Pathway Modulates Ca2+ Release and Muscle Contraction in Zebrafish Skeletal Muscle.Front Physiol. 2017 Aug 23;8:607. doi: 10.3389/fphys.2017.00607. eCollection 2017. Front Physiol. 2017. PMID: 28878687 Free PMC article.
-
Ca2+-activated Cl- channel TMEM16A/ANO1 identified in zebrafish skeletal muscle is crucial for action potential acceleration.Nat Commun. 2019 Jan 10;10(1):115. doi: 10.1038/s41467-018-07918-z. Nat Commun. 2019. PMID: 30631052 Free PMC article.
-
Ca(V)1.1: The atypical prototypical voltage-gated Ca²⁺ channel.Biochim Biophys Acta. 2013 Jul;1828(7):1587-97. doi: 10.1016/j.bbamem.2012.09.007. Epub 2012 Sep 13. Biochim Biophys Acta. 2013. PMID: 22982493 Free PMC article. Review.
References
-
- Liman, E. R., Hess, P., Weaver, F., and Koren, G. (1991) Nature 353 752-756 - PubMed
-
- Papazian, D. M., Timpe, L. C., Jan, Y. N., and Jan, L. Y. (1991) Nature 349 305-310 - PubMed
-
- Schneider, M. F., and Chandler, W. K. (1973) Nature 242 244-246 - PubMed
-
- Rios, E., and Brum, G. (1987) Nature 325 717-720 - PubMed
-
- Armstrong, C. M., Bezanilla, F. M., and Horowicz, P. (1972) Biochim. Biophys. Acta 267 605-608 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

