Switch 1 mutation S217A converts myosin V into a low duty ratio motor
- PMID: 19008235
- PMCID: PMC2629086
- DOI: 10.1074/jbc.M805530200
Switch 1 mutation S217A converts myosin V into a low duty ratio motor
Abstract
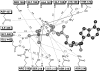
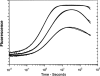
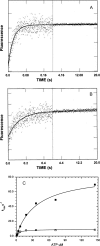
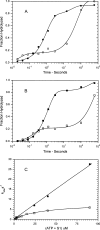
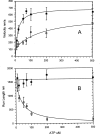
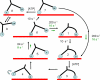
We have determined the kinetic mechanism and motile properties of the switch 1 mutant S217A of myosin Va. Phosphate dissociation from myosin V-ADP-Pi (inorganic phosphate) and actomyosin V-ADP-Pi and the rate of the hydrolysis step (myosin V-ATP-->myosin V-ADP-Pi) were all approximately 10-fold slower in the S217A mutant than in wild type (WT) myosin V, resulting in a slower steady-state rate of basal and filamentous actin (actin)-activated ATP hydrolysis. Substrate binding and ADP dissociation kinetics were all similar to or slightly faster in S217A than in WT myosin V and mechanochemical gating of the rates of dissociation of ADP between trail and lead heads is maintained. The reduction in the rate constants of the hydrolysis and phosphate dissociation steps reduces the duty ratio from approximately 0.85 in WT myosin V to approximately 0.25 in S217A and produces a motor in which the average run length on actin at physiological concentrations of ATP is reduced 10-fold. Thus we demonstrate that, by mutational perturbation of the switch 1 structure, myosin V can be converted into a low duty ratio motor that is processive only at low substrate concentrations.
Figures
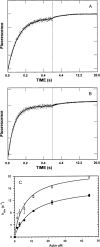
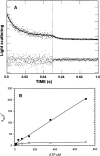
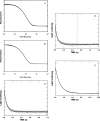
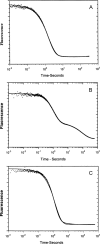
References
-
- Sellers, J. R., and Veigel, C. (2006) Curr. Opin. Cell Biol. 18 68-73 - PubMed
-
- Wu, X., Bowers, B., Wei, Q., Kocher, B., and Hammer, J. A., III (1997) J. Cell Sci. 110 847-859 - PubMed
-
- Mehta, A. D., Rock, R. S., Rief, M., Spudich, J. A., Mooseker, M. S., and Cheney, R. E. (1999) Nature 400 590-593 - PubMed
-
- Yildiz, A., Forkey, J. N., McKinney, S. A., Ha, T., Goldman, Y. E., and Selvin, P. R. (2003) Science 300 2061-2065 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

