Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial
- PMID: 19008268
- PMCID: PMC2583390
- DOI: 10.1136/bmj.a2052
Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial
Abstract
Objective: To compare the incidence of tuberculosis over two years in infants vaccinated at birth with intradermal BCG or with percutaneous BCG.
Design: Randomised trial.
Setting: South Africa.
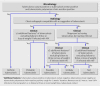
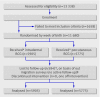
Participants: 11,680 newborn infants.
Interventions: Infants were randomised by week of birth to receive Tokyo 172 BCG vaccine through the percutaneous route (n=5775) or intradermal route (n=5905) within 24 hours of birth and followed up for two years.
Main outcome measures: The primary outcome measure was documented Mycobacterium tuberculosis infection or radiological and clinical evidence of tuberculosis disease. Secondary outcome measures were rates of adverse events, all cause and tuberculosis specific admissions to hospital, and mortality.
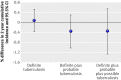
Results: The difference in the cumulative incidence of definite, probable, and possible tuberculosis between the intradermal group and the percutaneous group, as defined using study definitions based on microbiological, radiological, and clinical findings was -0.36% (95.5% confidence interval -1.27% to 0.54%). No significant differences were found between the routes in the cumulative incidence of tuberculosis using a range of equivalence of "within 25%." Additionally, no significant differences were found between the routes in the cumulative incidence of adverse events (risk ratio 0.98, 95% confidence interval 0.91 to 1.06), including deaths (1.19, 0.89 to 1.58).
Conclusion: Equivalence was found between intradermal BCG vaccine and percutaneous BCG in the incidence of tuberculosis in South African infants vaccinated at birth and followed up for two years. The World Health Organization should consider revising its policy of preferential intradermal vaccination to allow national immunisation programmes to choose percutaneous vaccination if that is more practical. Trial registration ClinicalTrials.gov NCT00242047.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
BCG vaccination in children.BMJ. 2008 Nov 13;337:a2086. doi: 10.1136/bmj.a2086. BMJ. 2008. PMID: 19008270 No abstract available.
References
-
- Maartens G, Wilkinson RJ. Tuberculosis. Lancet 2007. 15;370:2030-43. - PubMed
-
- World Health Organization. BCG vaccine WHO position paper. Wkly Epidemiol Rec 2004;79:27-38. - PubMed
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994;2;271:698-702. - PubMed
-
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993;22:1154-8. - PubMed
-
- Bricks LF. Percutaneous or intradermal BCG vaccine? J Pediatr (Rio J) 2004;80:93-8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
