Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice
- PMID: 19008300
- PMCID: PMC2722216
- DOI: 10.1093/hmg/ddn386
Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice
Abstract
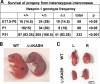
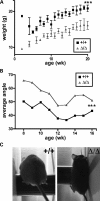
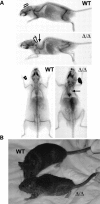
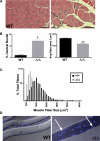
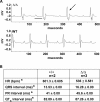
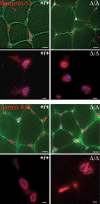
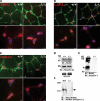
Mutations in the gene encoding the inner nuclear membrane proteins lamins A and C produce cardiac and skeletal muscle dysfunction referred to as Emery Dreifuss muscular dystrophy. Lamins A and C participate in the LINC complex that, along with the nesprin and SUN proteins, LInk the Nucleoskeleton with the Cytoskeleton. Nesprins 1 and 2 are giant spectrin-repeat containing proteins that have large and small forms. The nesprins contain a transmembrane anchor that tethers to the nuclear membrane followed by a short domain that resides within the lumen between the inner and outer nuclear membrane. Nesprin's luminal domain binds directly to SUN proteins. We generated mice where the C-terminus of nesprin-1 was deleted. This strategy produced a protein lacking the transmembrane and luminal domains that together are referred to as the KASH domain. Mice homozygous for this mutation exhibit lethality with approximately half dying at or near birth from respiratory failure. Surviving mice display hindlimb weakness and an abnormal gait. With increasing age, kyphoscoliosis, muscle pathology and cardiac conduction defects develop. The protein components of the LINC complex, including mutant nesprin-1alpha, lamin A/C and SUN2, are localized at the nuclear membrane in this model. However, the LINC components do not normally associate since coimmunoprecipitation experiments with SUN2 and nesprin reveal that mutant nesprin-1 protein no longer interacts with SUN2. These findings demonstrate the role of the LINC complex, and nesprin-1, in neuromuscular and cardiac disease.
Figures



References
-
- Favreau C., Dubosclard E., Ostlund C., Vigouroux C., Capeau J., Wehnert M., Higuet D., Worman H.J., Courvalin J.C., Buendia B. Expression of lamin A mutated in the carboxyl-terminal tail generates an aberrant nuclear phenotype similar to that observed in cells from patients with Dunnigan-type partial lipodystrophy and Emery-Dreifuss muscular dystrophy. Exp. Cell Res. 2003;282:14–23. - PubMed
-
- Bonne G., Mercuri E., Muchir A., Urtizberea A., Becane H.M., Recan D., Merlini L., Wehnert M., Boor R., Reuner U., et al. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann. Neurol. 2000;48:170–180. - PubMed
-
- Wang Y., Herron A.J., Worman H.J. Pathology and nuclear abnormalities in hearts of transgenic mice expressing M371K lamin A encoded by an LMNA mutation causing Emery-Dreifuss muscular dystrophy. Hum. Mol. Genet. 2006;15:2479–2489. - PubMed
-
- Arimura T., Helbling-Leclerc A., Massart C., Varnous S., Niel F., Lacene E., Fromes Y., Toussaint M., Mura A.M., Keller D.I., et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 2005;14:155–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

