Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses
- PMID: 19008313
- PMCID: PMC2654731
- DOI: 10.1210/en.2008-0293
Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses
Abstract
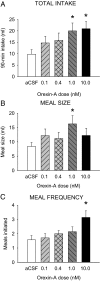
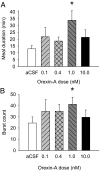
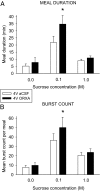
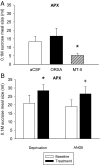
Orexin-A (ORXA) is an orexigenic neuropeptide produced by the lateral hypothalamus that increases food intake when injected into the brain ventricles or forebrain nuclei. We used a licking microstructure analysis to evaluate hindbrain and forebrain ORXA effects in intact and hindbrain-lesioned rats, to identify the motivational and anatomical bases of ORXA hyperphagia. Intact rats with cannulas in the fourth brain ventricle (4V) received vehicle (artificial cerebrospinal fluid) or ORXA (0.1, 0.4, 1, or 10 nm) injections before 90 min access to 0.1 m sucrose. Meal size and frequency were increased in a double-dissociated manner by the 1 and 10 nm doses, respectively. In experiment 2, 4V 1 nm ORXA was applied to rats offered solutions varied in caloric and gustatory intensity (water and 0.1 and 1 m sucrose). ORXA increased meal frequency for all tastants. ORXA increased meal size only for 0.1 m sucrose, by prolonging the meal without affecting early ingestion rate or lick burst size, suggesting that 4V ORXA influenced inhibitory postingestive feedback rather than taste evaluation. In experiment 3, rats with cannulas in the third ventricle (3V) received dorsal medullary lesions centered on the area postrema (APX group) or sham procedures, and licking for water and 0.1 and 1 m sucrose was evaluated after 1 nm 3V ORXA/artificial cerebrospinal fluid injections. The 3V ORXA increased 0.1 m sucrose meal size and meal frequency for all tastants in the sham group, as observed after 4V ORXA in experiment 2. In the APX group, 3V ORXA injections influenced meal frequency, but they no longer increased meal size. However, the APX rats increased meal size for 0.1 m sucrose after food and water deprivation and after 3V angiotensin II injection. They also showed meal size suppression after 3V injection of the melanocortin-3/4 receptor agonist melanotan II (1 nm). These findings suggest that the area postrema and subjacent nucleus of the solitary tract are necessary for increases in consummatory (meal size) but not appetitive (meal frequency) responses to 3V ORXA. The meal size increases may be due to reduced postingestive feedback inhibition induced by ORXA delivered to either the hindbrain or forebrain ventricles.
Figures


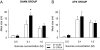
References
-
- Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T 1999 Orexins/hypocretins regulate drinking behaviour. Brain Res 842:256–261 - PubMed
-
- Krowicki ZK, Burmeister MA, Berthoud HR, Scullion RT, Fuchs K, Hornby PJ 2002 Orexins in rat dorsal motor nucleus of the vagus potently stimulate gastric motor function. Am J Physiol Gastrointest Liver Physiol 283:G465–G472 - PubMed
-
- Miyasaka K, Masuda M, Kanai S, Sato N, Kurosawa M, Funakoshi A 2002 Central orexin-A stimulates pancreatic exocrine secretion via the vagus. Pancreas 25:400–404 - PubMed
-
- Rodgers RJ, Ishii Y, Halford JC, Blundell JE 2002 Orexins and appetite regulation. Neuropeptides 36:303–325 - PubMed
-
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M 1998 Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:1, page following 696 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

