Tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis by the expansion of CD4+CD25+ regulatory T cells
- PMID: 19008314
- PMCID: PMC2659286
- DOI: 10.1210/en.2008-1389
Tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis by the expansion of CD4+CD25+ regulatory T cells
Abstract
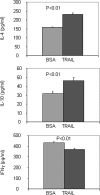
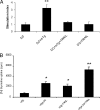
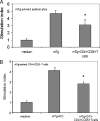
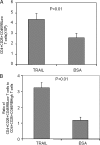
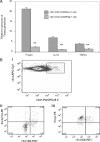
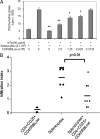
There have been several reports that TNF-related apoptosis-inducing ligand (TRAIL) has the ability to suppress the development of experimental autoimmune diseases, including a mouse model of experimental autoimmune encephalomyelitis, a rabbit model of rheumatoid arthritis, type 1 diabetes mellitus, in mice and experimental autoimmune thyroiditis (EAT) in mice. However, the mechanism underlying TRAIL effect is not well defined. In the present study, we specifically examined TRAIL effects on CD4(+)CD25(+) regulatory T cells. CD4(+)CD25(+) T cells prepared from mouse thyroglobulin (mTg)-immunized CBA/J mice proliferate in the presence of TRAIL and dendritic cells in vitro. These CD4(+)CD25(+) T cells included both CD4(+)CD25(+)CD45RB(Low) (regulatory) and CD4(+)CD25(+)CD45RB(High) (effector) T cells. Our results demonstrated that mTg-immunized mice treated with TRAIL showed significant increases in the number of CD4(+)CD25(+)CD45RB(Low) T cells compared with mice immunized with mTg alone. CD4(+)CD25(+)CD45RB(Low) T cells expressed much higher levels of the forkhead family transcription factor, IL-10, and TGFbeta1 than CD4(+)CD25(+)CD45RB(High) T cells, and these cells can completely suppress the proliferation of the mTg-primed splenocytes in lower concentrations than the unfractionated CD4(+)CD25(+) T cells. Furthermore, transfer of these cells into CBA/J mice prior to mTg-primed splenocyte injection could markedly reduce the frequency and severity of EAT development. CD4(+)CD25(+)CD45RB(Low) T cells were more effective at suppressing histological thyroiditis than unfractionated cells. These results indicated that TRAIL can increase the number of mTg-specific CD4(+)CD25(+)CD45RB(Low) T cells, inhibiting autoimmune responses and preventing the progression of EAT. These findings reveal a novel mechanism by which TRAIL could inhibit autoimmune disease.
Figures
Similar articles
-
IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis.J Immunol. 2005 Jun 1;174(11):7006-13. doi: 10.4049/jimmunol.174.11.7006. J Immunol. 2005. PMID: 15905543
-
Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis.J Immunol. 2003 Jun 1;170(11):5511-22. doi: 10.4049/jimmunol.170.11.5511. J Immunol. 2003. PMID: 12759428
-
Naturally-existing CD4(+)CD25(+)Foxp3(+) regulatory T cells are required for tolerance to experimental autoimmune thyroiditis induced by either exogenous or endogenous autoantigen.J Autoimmun. 2009 Aug;33(1):68-76. doi: 10.1016/j.jaut.2009.03.010. Epub 2009 Apr 17. J Autoimmun. 2009. PMID: 19375891 Free PMC article.
-
CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects.Am J Transplant. 2007 Jun;7(6):1457-63. doi: 10.1111/j.1600-6143.2007.01829.x. Am J Transplant. 2007. PMID: 17511675 Review.
-
The role of CD4+CD25+ T regulatory cells in autoimmune diseases.Clin Rev Allergy Immunol. 2008 Jun;34(3):338-44. doi: 10.1007/s12016-007-8043-0. Clin Rev Allergy Immunol. 2008. PMID: 18092144 Review.
Cited by
-
Gender-Specific Impact of Metabolic Obesity Phenotypes on the Risk of Hashimoto's Thyroiditis: A Retrospective Data Analysis Using a Health Check-Up Database.J Inflamm Res. 2022 Feb 7;15:827-837. doi: 10.2147/JIR.S353384. eCollection 2022. J Inflamm Res. 2022. PMID: 35173456 Free PMC article.
-
RAGE is a critical factor of sex-based differences in age-induced kidney damage.Front Physiol. 2023 Mar 29;14:1154551. doi: 10.3389/fphys.2023.1154551. eCollection 2023. Front Physiol. 2023. PMID: 37064891 Free PMC article.
-
The Association of Obesity with Autoimmune Thyroiditis and Thyroid Function-Possible Mechanisms of Bilateral Interaction.Int J Endocrinol. 2020 Dec 14;2020:8894792. doi: 10.1155/2020/8894792. eCollection 2020. Int J Endocrinol. 2020. PMID: 33381173 Free PMC article. Review.
-
Mucopolysaccharide diseases: a complex interplay between neuroinflammation, microglial activation and adaptive immunity.J Inherit Metab Dis. 2014 Jan;37(1):1-12. doi: 10.1007/s10545-013-9613-3. Epub 2013 May 8. J Inherit Metab Dis. 2014. PMID: 23653226 Review.
-
Overexpression of BID in thyroids of transgenic mice increases sensitivity to iodine-induced autoimmune thyroiditis.J Transl Med. 2014 Jun 23;12:180. doi: 10.1186/1479-5876-12-180. J Transl Med. 2014. PMID: 24957380 Free PMC article.
References
-
- Wang SH, Bretz JD, Phelps E, Mezosi E, Arscott PL, Utsugi S, Baker Jr JR 2002 A unique combination of inflammatory cytokines enhances apoptosis of thyroid follicular cells and transforms nondestructive to destructive thyroiditis in experimental autoimmune thyroiditis. J Immunol 168:2470–2474 - PubMed
-
- Mezosi E, Wang SH, Utsugi S, Bajnok L, Bretz JD, Gauger PG, Thompson NW, Baker Jr JR 2005 Induction and regulation of Fas-mediated apoptosis in human thyroid epithelial cells. Mol Endocrinol 19:804–811 - PubMed
-
- Wang SH, Van Antwerp M, Kuick R, Gauger PG, Doherty GM, Fan YY, Baker Jr JR 2007 Microarray analysis of cytokine activation of apoptosis pathways in the thyroid. Endocrinology 148:4844–4852 - PubMed
-
- Baecher-Allan C, Hafler DA 2006 Human regulatory T cells and their role in autoimmune disease. Immunol Rev 212:203–216 - PubMed
-
- Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM 2007 FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol 178:2458–2468 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

