Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway
- PMID: 19008341
- PMCID: PMC2628607
- DOI: 10.2337/db07-1795
Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway
Abstract
Objective: SIRT1, a class III histone/protein deacetylase, is known to interfere with the nuclear factor-kappaB (NF-kappaB) signaling pathway and thereby has an anti-inflammatory function. Because of the central role of NF-kappaB in cytokine-mediated pancreatic beta-cell damage, we postulated that SIRT1 might work in pancreatic beta-cell damage models.
Research design and methods: RINm5F (RIN) cells or isolated rat islets were treated with interleukin-1beta and interferon-gamma. SIRT1 was activated by resveratrol, a pharmacological activator, or ectopic overexpression. The underlying mechanisms of SIRT1 against cytokine toxicity were further explored.
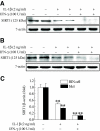
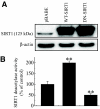
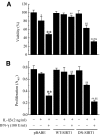
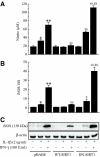
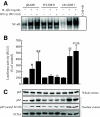
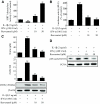
Results: Treatment of RIN cells with cytokines induced cell damage, and this damage was well correlated with the expression of the inducible form of nitric oxide (NO) synthase (iNOS) and NO production. However, SIRT1 overexpression completely prevented cytokine-mediated cytotoxicity, NO production, and iNOS expression. The molecular mechanism by which SIRT1 inhibits iNOS expression appeared to involve the inhibition of the NF-kappaB signaling pathway through deacetylation of p65. In addition, SIRT1 activation by either resveratrol or adenoviral-directed overexpression of SIRT1 could prevent cytokine toxicity and maintain normal insulin-secreting responses to glucose in isolated rat islets.
Conclusions: This study will provide valuable information not only into the mechanisms underlying beta-cell destruction but also into the regulation of SIRT1 as a possible target to attenuate cytokine-induced beta-cell damage.
Figures

References
-
- Cetkovic-Cvrlje M, Eizirik DL: TNF-α and IFN-γ potentiate the deleterious effects of IL-1β on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine 6: 399–406, 1994 - PubMed
-
- Heitmeier MR, Scarim AL, Corbett JA: Interferon-γ increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem 272: 13697–13704, 1997 - PubMed
-
- Baeuerle PA, Henkel T: Function and activation of NF-κB in the immune system. Annu Rev Immunol 12: 141–179, 1994 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

