The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha
- PMID: 19008357
- PMCID: PMC2584738
- DOI: 10.1073/pnas.0804446105
The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha
Abstract
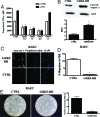
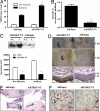
G-protein-coupled receptor (GPCR) kinases, GRKs, are known as serine/threonine kinases that regulate GPCR signaling, but recent findings propose functions for these kinases besides receptor desensitization. Indeed, GRK5 can translocate to the nucleus by means of a nuclear localization sequence, suggesting that this kinase regulates transcription events in the nucleus. To evaluate the effect of GRK5-IkappaB alpha interaction on NFkappaB signaling, we induced the overexpression and the knockdown of GRK5 in cell cultures. GRK5 overexpression causes nuclear accumulation of IkappaB alpha, leading to the inhibition of NFkappaB transcriptional activity. Opposite results are achieved by GRK5 knockdown through siRNA. A physical interaction between GRK5 and IkappaB alpha, rather than phosphorylative events, appears as the underlying mechanism. We identify the regulator of gene protein signaling homology domain of GRK5 (RH) and the N-terminal domain of IkappaB alpha as the regions involved in such interaction. To confirm the biological relevance of this mechanism of regulation for NFkappaB, we evaluated the effects of GRK5-RH on NFkappaB-dependent phenotypes. In particular, GRK5-RH overexpression impairs apoptosis protection and cytokine production in vitro and inflammation and tissue regeneration in vivo. Our results reveal an unexpected role for GRK5 in the regulation of NFkappaB transcription activity. Placing these findings in perspective, this mechanism may represent a therapeutic target for all those conditions involving excessive NFkappaB activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Birbach A, et al. Signaling molecules of the NF-kappa B pathway shuttle constitutively between cytoplasm and nucleus. J Biol Chem. 2002;277:10842–10851. - PubMed
-
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. - PubMed
-
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. - PubMed
-
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. - PubMed
-
- Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

