Overexpression of apolipoprotein F reduces HDL cholesterol levels in vivo
- PMID: 19008531
- PMCID: PMC2766561
- DOI: 10.1161/ATVBAHA.108.177105
Overexpression of apolipoprotein F reduces HDL cholesterol levels in vivo
Abstract
Objective: Apolipoprotein F (ApoF) is a protein component of several lipoprotein classes including HDL. It is also known as lipid transfer inhibitor protein (LTIP) based on its ability to inhibit lipid transfer between lipoproteins ex vivo. We sought to investigate the role of ApoF in HDL metabolism.
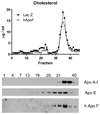
Methods and results: Adeno-associated viruses (AAV) based on serotype 8, were used to overexpress either murine or human ApoF in mice. Overexpression of murine ApoF significantly reduced total cholesterol levels by 28% (P<0.001), HDL by 27% (P<0.001), and phospholipid levels by 19% (P<0.001). Overexpression of human ApoF had similar effects. Human ApoF was nearly exclusively HDL-associated in mice. In agreement with this finding, greater than 90% of the ApoF in human plasma was found on HDL(3), with only a small amount on LDL. Overexpression of mouse ApoF accelerated the plasma clearance of [(3)H]-cholesteryl ether labeled HDL. Plasma from mice overexpressing ApoF showed improved macrophage cholesterol efflux on a per HDL-C basis.
Conclusions: ApoF overexpression reduces HDL cholesterol levels in mice by increasing clearance of HDL-CE. ApoF may be an important determinant of HDL metabolism and reverse cholesterol transport.
Figures




References
-
- Koren E, McConathy WJ, Alaupovic P. Isolation and characterization of simple and complex lipoproteins containing apolipoprotein F from human plasma. Biochemistry. 1982;21:5347–5351. - PubMed
-
- Olofsson SO, McConathy WJ, Alaupovic P. Isolation and partial characterization of a new acidic apolipoprotein (apolipoprotein F) from high density lipoproteins of human plasma. Biochemistry. 1978;17:1032–1036. - PubMed
-
- Day JR, Albers JJ, Gilbert TL, Whitmore TE, McConathy WJ, Wolfbauer G. Purification and molecular cloning of human apolipoprotein F. Biochem Biophys Res Commun. 1994;203:1146–1151. - PubMed
-
- Wang X, Driscoll DM, Morton RE. Molecular cloning and expression of lipid transfer inhibitor protein reveals its identity with apolipoprotein F. J Biol Chem. 1999;274:1814–1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

