Auto-activation mechanism of the Mycobacterium tuberculosis PknB receptor Ser/Thr kinase
- PMID: 19008858
- PMCID: PMC2599879
- DOI: 10.1038/emboj.2008.236
Auto-activation mechanism of the Mycobacterium tuberculosis PknB receptor Ser/Thr kinase
Abstract
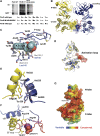
Many Ser/Thr protein kinases are activated by autophosphorylation, but the mechanism of this process has not been defined. We determined the crystal structure of a mutant of the Ser/Thr kinase domain (KD) of the mycobacterial sensor kinase PknB in complex with an ATP competitive inhibitor and discovered features consistent with an activation complex. The complex formed an asymmetric dimer, with the G helix and the ordered activation loop of one KD in contact with the G helix of the other. The activation loop of this putative 'substrate' KD was disordered, with the ends positioned at the entrance to the partner KD active site. Single amino-acid substitutions in the G-helix interface reduced activation-loop phosphorylation, and multiple replacements abolished KD phosphorylation and kinase activation. Phosphorylation of an inactive mutant KD was reduced by G-helix substitutions in both active and inactive KDs, as predicted by the idea that the asymmetric dimer mimics a trans-autophosphorylation complex. These results support a model in which a structurally and functionally asymmetric, 'front-to-front' association mediates autophosphorylation of PknB and homologous kinases.
Figures




Similar articles
-
Allosteric activation by dimerization of the PknD receptor Ser/Thr protein kinase from Mycobacterium tuberculosis.J Biol Chem. 2007 Apr 13;282(15):11427-35. doi: 10.1074/jbc.M610193200. Epub 2007 Jan 22. J Biol Chem. 2007. PMID: 17242402
-
Allosteric activation mechanism of the Mycobacterium tuberculosis receptor Ser/Thr protein kinase, PknB.Structure. 2010 Dec 8;18(12):1667-77. doi: 10.1016/j.str.2010.09.019. Structure. 2010. PMID: 21134645 Free PMC article.
-
Mechanism of autophosphorylation of mycobacterial PknB explored by molecular dynamics simulations.Biochemistry. 2014 Jul 22;53(28):4715-26. doi: 10.1021/bi500245v. Epub 2014 Jul 14. Biochemistry. 2014. PMID: 24988180
-
Signaling mechanisms of the Mycobacterium tuberculosis receptor Ser/Thr protein kinases.Curr Opin Struct Biol. 2009 Dec;19(6):650-7. doi: 10.1016/j.sbi.2009.10.017. Epub 2009 Nov 14. Curr Opin Struct Biol. 2009. PMID: 19914822 Free PMC article. Review.
-
Mycobacterial Ser/Thr protein kinases and phosphatases: physiological roles and therapeutic potential.Biochim Biophys Acta. 2008 Jan;1784(1):193-202. doi: 10.1016/j.bbapap.2007.08.006. Epub 2007 Aug 14. Biochim Biophys Acta. 2008. PMID: 17869195 Review.
Cited by
-
New Test System for Serine/Threonine Protein Kinase Inhibitors Screening: E. coli APHVIII/Pk25 design.Acta Naturae. 2010 Jul;2(3):110-21. Acta Naturae. 2010. PMID: 22649658 Free PMC article.
-
Understanding the role of PknJ in Mycobacterium tuberculosis: biochemical characterization and identification of novel substrate pyruvate kinase A.PLoS One. 2010 May 24;5(5):e10772. doi: 10.1371/journal.pone.0010772. PLoS One. 2010. PMID: 20520732 Free PMC article.
-
Eukaryote-like serine/threonine kinases and phosphatases in bacteria.Microbiol Mol Biol Rev. 2011 Mar;75(1):192-212. doi: 10.1128/MMBR.00042-10. Microbiol Mol Biol Rev. 2011. PMID: 21372323 Free PMC article. Review.
-
Growth- and Stress-Induced PASTA Kinase Phosphorylation in Enterococcus faecalis.J Bacteriol. 2017 Oct 3;199(21):e00363-17. doi: 10.1128/JB.00363-17. Print 2017 Nov 1. J Bacteriol. 2017. PMID: 28808126 Free PMC article.
-
Mycobacterium tuberculosis Serine/Threonine Protein Kinases.Microbiol Spectr. 2014 Oct;2(5):10.1128/microbiolspec.MGM2-0006-2013. doi: 10.1128/microbiolspec.MGM2-0006-2013. Microbiol Spectr. 2014. PMID: 25429354 Free PMC article. Review.
References
-
- Av-Gay Y, Everett M (2000) The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol 8: 238–244 - PubMed
-
- Boitel B, Ortiz-Lombardia M, Duran R, Pompeo F, Cole ST, Cervenansky C, Alzari PM (2003) PknB kinase activity is regulated by phosphorylation in two Thr residues and dephosphorylation by PstP, the cognate phospho-Ser/Thr phosphatase, in Mycobacterium tuberculosis. Mol Microbiol 49: 1493–1508 - PubMed
-
- Dar AC, Dever TE, Sicheri F (2005) Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell 122: 887–900 - PubMed
-
- DeLano WL (2002) The PyMOL Molecular Graphics System. San Carlos, CA: DeLano Scientific
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

