Determination of bacterial rod shape by a novel cytoskeletal membrane protein
- PMID: 19008860
- PMCID: PMC2599877
- DOI: 10.1038/emboj.2008.234
Determination of bacterial rod shape by a novel cytoskeletal membrane protein
Abstract
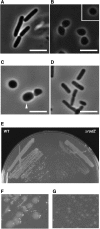
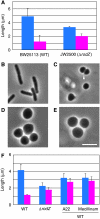
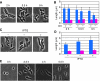
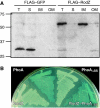
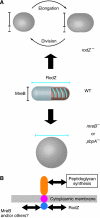
Cell shape is critical for growth, and some genes are involved in bacterial cell morphogenesis. Here, we report a novel gene, rodZ, required for the determination of rod shape in Escherichia coli. Cells lacking rodZ no longer had rod shape but rather were round or oval. These round cells were smaller than known round mutant cells, including mreB and pbpA mutants; both are known to lose rod shape. Morphogenesis from rod cells to round cells and vice versa, caused by depletion and overproduction of RodZ, respectively, revealed that RodZ could regulate the length of the long axis of the cell. RodZ is a membrane protein with bitopic topology such that the N-terminal region including a helix-turn-helix motif is in the cytoplasm, whereas the C-terminal region is exposed in the periplasm. GFP-RodZ forms spirals along the lateral axis of the cell beneath the cell membrane, similar to the MreB bacterial actin. Thus, RodZ may mediate spatial information from cytoskeletal proteins in the cytoplasm to a peptidoglycan synthesis machinery in the periplasm.
Figures

Similar articles
-
[Regulation of determination of bacterial shape].Nihon Saikingaku Zasshi. 2014;69(4):557-64. doi: 10.3412/jsb.69.557. Nihon Saikingaku Zasshi. 2014. PMID: 25447981 Review. Japanese.
-
The periplasmic disordered domain of RodZ promotes its self-interaction in Escherichia coli.Genes Cells. 2018 Apr;23(4):307-317. doi: 10.1111/gtc.12572. Epub 2018 Feb 26. Genes Cells. 2018. PMID: 29480545
-
Chlamydial MreB Directs Cell Division and Peptidoglycan Synthesis in Escherichia coli in the Absence of FtsZ Activity.mBio. 2020 Feb 18;11(1):e03222-19. doi: 10.1128/mBio.03222-19. mBio. 2020. PMID: 32071268 Free PMC article.
-
Mutations in cell elongation genes mreB, mrdA and mrdB suppress the shape defect of RodZ-deficient cells.Mol Microbiol. 2013 Mar;87(5):1029-44. doi: 10.1111/mmi.12148. Epub 2013 Jan 21. Mol Microbiol. 2013. PMID: 23301723 Free PMC article.
-
RodZ: a key-player in cell elongation and cell division in Escherichia coli.AIMS Microbiol. 2019 Nov 7;5(4):358-367. doi: 10.3934/microbiol.2019.4.358. eCollection 2019. AIMS Microbiol. 2019. PMID: 31915748 Free PMC article. Review.
Cited by
-
Engineering AraC to make it responsive to light instead of arabinose.Nat Chem Biol. 2021 Jul;17(7):817-827. doi: 10.1038/s41589-021-00787-6. Epub 2021 Apr 26. Nat Chem Biol. 2021. PMID: 33903769
-
Mutations in the Lipopolysaccharide biosynthesis pathway interfere with crescentin-mediated cell curvature in Caulobacter crescentus.J Bacteriol. 2010 Jul;192(13):3368-78. doi: 10.1128/JB.01371-09. Epub 2010 Apr 30. J Bacteriol. 2010. PMID: 20435724 Free PMC article.
-
Sensitivity of Deinococcus grandis rodZ deletion mutant to calcium ions results in enhanced spheroplast size.AIMS Microbiol. 2019 Jun 26;5(2):176-185. doi: 10.3934/microbiol.2019.2.176. eCollection 2019. AIMS Microbiol. 2019. PMID: 31384711 Free PMC article.
-
RodZ, a new player in bacterial cell morphogenesis.EMBO J. 2009 Feb 4;28(3):171-2. doi: 10.1038/emboj.2008.287. EMBO J. 2009. PMID: 19194484 Free PMC article. No abstract available.
-
RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli.EMBO J. 2009 Feb 4;28(3):193-204. doi: 10.1038/emboj.2008.264. Epub 2008 Dec 11. EMBO J. 2009. PMID: 19078962 Free PMC article.
References
-
- Ausmees N, Kuhn JR, Jacobs-Wagner C (2003) The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell 115: 705–713 - PubMed
-
- Carballido-Lopez R, Formstone A (2007) Shape determination in Bacillus subtilis. Curr Opin Microbiol 10: 611–616 - PubMed
-
- Carballido-Lopez R, Formstone A, Li Y, Ehrlich SD, Noirot P, Errington J (2006) Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev Cell 11: 399–409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

