Malleable machines take shape in eukaryotic transcriptional regulation
- PMID: 19008886
- PMCID: PMC2921704
- DOI: 10.1038/nchembio.127
Malleable machines take shape in eukaryotic transcriptional regulation
Abstract
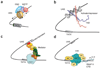
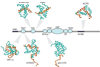
Transcriptional control requires the spatially and temporally coordinated action of many macromolecular complexes. Chromosomal proteins, transcription factors, co-activators and components of the general transcription machinery, including RNA polymerases, often use structurally or stoichiometrically ill-defined regions for interactions that convey regulatory information in processes ranging from chromatin remodeling to mRNA processing. Determining the functional significance of intrinsically disordered protein regions and developing conceptual models of their action will help to illuminate their key role in transcription regulation. Complexes comprising disordered regions often display short recognition elements embedded in flexible and sequentially variable environments that can lead to structural and functional malleability. This provides versatility to recognize multiple targets having different structures, facilitate conformational rearrangements and physically communicate with many partners in response to environmental changes. All these features expand the capacities of ordered complexes and give rise to efficient regulatory mechanisms.
Figures
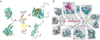
References
-
- Sigler PB. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988;333:210–212. - PubMed
-
- Dunker AK, Obradovic Z, Romero P, Garner EC, Brown CJ. Intrinsic protein disorder in complete genomes. Genome Inform. Ser. Workshop Genome Inform. 2000;11:161–171. - PubMed
-
- Asturias FJ. Another piece in the transcription initiation puzzle. Nat. Struct. Mol. Biol. 2004;11:1031–1033. - PubMed
-
- Romero P, et al. Thousands of proteins likely to have long disordered regions. Pac. Symp. Biocomput. 1998;3:437–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

