A generic mechanism of emergence of amyloid protofilaments from disordered oligomeric aggregates
- PMID: 19008938
- PMCID: PMC2572140
- DOI: 10.1371/journal.pcbi.1000222
A generic mechanism of emergence of amyloid protofilaments from disordered oligomeric aggregates
Abstract
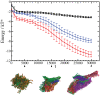
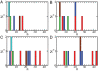
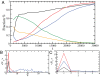
The presence of oligomeric aggregates, which is often observed during the process of amyloid formation, has recently attracted much attention because it has been associated with a range of neurodegenerative conditions including Alzheimer's and Parkinson's diseases. We provide a description of a sequence-indepedent mechanism by which polypeptide chains aggregate by forming metastable oligomeric intermediate states prior to converting into fibrillar structures. Our results illustrate that the formation of ordered arrays of hydrogen bonds drives the formation of beta-sheets within the disordered oligomeric aggregates that form early under the effect of hydrophobic forces. Individual beta-sheets initially form with random orientations and subsequently tend to align into protofilaments as their lengths increase. Our results suggest that amyloid aggregation represents an example of the Ostwald step rule of first-order phase transitions by showing that ordered cross-beta structures emerge preferentially from disordered compact dynamical intermediate assemblies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

