Zebrafish mutants calamity and catastrophe define critical pathways of gene-nutrient interactions in developmental copper metabolism
- PMID: 19008952
- PMCID: PMC2576455
- DOI: 10.1371/journal.pgen.1000261
Zebrafish mutants calamity and catastrophe define critical pathways of gene-nutrient interactions in developmental copper metabolism
Abstract
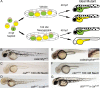
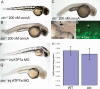
Nutrient availability is an important environmental variable during development that has significant effects on the metabolism, health, and viability of an organism. To understand these interactions for the nutrient copper, we used a chemical genetic screen for zebrafish mutants sensitive to developmental copper deficiency. In this screen, we isolated two mutants that define subtleties of copper metabolism. The first contains a viable hypomorphic allele of atp7a and results in a loss of pigmentation when exposed to mild nutritional copper deficiency. This mutant displays incompletely penetrant skeletal defects affected by developmental copper availability. The second carries an inactivating mutation in the vacuolar ATPase that causes punctate melanocytes and embryonic lethality. This mutant, catastrophe, is sensitive to copper deprivation revealing overlap between ion metabolic pathways. Together, the two mutants illustrate the utility of chemical genetic screens in zebrafish to elucidate the interaction of nutrient availability and genetic polymorphisms in cellular metabolism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. Am J Epidemiol. 1998;147:213–216. - PubMed
-
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. - PubMed
-
- Menkes JH. Kinky hair disease: twenty five years later. Brain Dev. 1988;10:77–79. - PubMed
-
- Menkes JH, Alter M, Steigleder GK, Weakley DR, Sung JH. A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics. 1962;29:764–779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

