Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts
- PMID: 19010455
- PMCID: PMC2655212
- DOI: 10.1016/j.bone.2008.10.037
Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts
Abstract
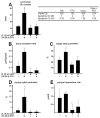
Intermittent administration of parathyroid hormone (PTH) stimulates bone formation on the surface of cancellous and periosteal bone by increasing the number of osteoblasts. Previous studies of ours in mice demonstrated that intermittent PTH increases cancellous osteoblast number at least in part by attenuating osteoblast apoptosis, but the mechanism responsible for the anabolic effect of the hormone on periosteal bone is unknown. We report that daily injections of 100 ng/g of PTH(1-34) to 4-6 month old mice increased the number of osteoblasts on the periosteum of lumbar vertebrae by 2-3 fold as early as after 2 days. However, the prevalence of apoptotic periosteal osteoblasts was only 0.2% in vehicle treated animals, which is approximately 20-fold lower than is the case for cancellous osteoblasts. Moreover, PTH did not have a discernable effect on periosteal osteoblast apoptosis. Administration of BrdU for 4 days failed to label periosteal osteoblasts under either basal conditions or following administration of PTH. Cancellous osteoblasts, on the other hand, were labeled under basal conditions, but PTH did not increase the percentage of BrdU-positive cells. Thus, intermittent PTH does not increase cancellous or periosteal osteoblast number by stimulating the proliferation of osteoblast progenitors. Consistent with high turnover of cancellous osteoblasts as compared to that of periosteal osteoblasts, ganciclovir-induced ablation of replicating osteoblast progenitors in mice expressing thymidine kinase under the control of the 3.6 kb rat Col1A1 promoter resulted in disappearance of osteoblasts from cancellous bone over a 7-14 day period, whereas periosteal osteoblasts were unaffected. However, 14 days of pre-treatment with ganciclovir prevented PTH anabolism on periosteal bone. We conclude that in cancellous bone, attenuation of osteoblast apoptosis by PTH increases osteoblast number because their rate of apoptosis is high, making this effect of the hormone profound. However, in periosteal bone where the rate of osteoblast apoptosis is low, PTH must exert pro-differentiating and/or pro-survival effects on post-mitotic pre-osteoblasts. Targeting the latter cells is an effective mechanism for increasing osteoblast number in periosteal bone where the production of osteoblasts from replicating progenitors is slow.
Figures





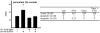
References
-
- Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007;40:1447–52. - PubMed
-
- Hodsman AB, Bauer DC, Dempster D, Dian L, Hanley DA, Harris ST, Kendler D, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. - PubMed
-
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. - PubMed
-
- Iida-Klein A, Lu SS, Cosman F, Lindsay R, Dempster DW. Effects of cyclic vs. daily treatment with human parathyroid hormone (1–34) on murine bone structure and cellular activity. Bone. 2007;40:391–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

