Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment
- PMID: 19010456
- PMCID: PMC2700945
- DOI: 10.1016/j.bone.2008.10.042
Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment
Abstract
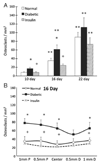
Fracture healing in diabetic individuals and in animal models of diabetes is impaired. To investigate mechanisms by which diabetes may affect fracture healing we focused on the transition from cartilage to bone, a midpoint in the fracture healing process. Femoral fractures were induced in mice rendered diabetic by multiple low dose streptozotocin treatment and compared to matching normoglycemic mice. One group of diabetic animals was treated with slow release insulin to maintain normal serum glucose levels. The results indicate that there was relatively little difference in the initial formation of the fracture callus on day 10. However, on day 16 the diabetic group had significantly smaller callus, greater loss of cartilage and enhanced osteoclastogenesis that was normalized by treatment with insulin when assessed by histomorphometric analysis. Chondrocyte apoptosis was significantly higher in diabetic mice and this increase was blocked by insulin. These changes were accompanied by diabetes-increased mRNA levels of RANKL, TNF-alpha, and ADAMTS-4 and -5 measured by real-time PCR, which was reversed by insulin treatment. On days 16 and 22 bone formation within the callus of diabetic mice was significantly less than the normoglycemic and brought to normal levels by insulin treatment. These results suggest that a significant effect of diabetes on fracture healing is increased chondrocyte apoptosis and osteoclastogenesis that accelerates the loss of cartilage and reduces the anlage for endochondral bone formation during fracture repair. That insulin reverses these effects demonstrates that they are directly related to the diabetic condition.
Figures






References
-
- Gerstenfeld LC, Wronski TJ, Hollinger JO, Einhorn TA. Application of histomorphometric methods to the study of bone repair. J Bone Miner Res. 2005;20:1715–1722. - PubMed
-
- Jehle PM, Jehle DR, Mohan S, Bohm BO. Serum levels of insulin-like growth factor system components and relationship to bone metabolism in Type 1 and Type 2 diabetes mellitus patients. J Endocrinol. 1998;159:297–306. - PubMed
-
- Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. - PubMed
-
- Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care. 2001;24:1198–1203. - PubMed
-
- Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24:1192–1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

