Self-assembling materials for therapeutic delivery
- PMID: 19010748
- PMCID: PMC2729065
- DOI: 10.1016/j.actbio.2008.09.018
Self-assembling materials for therapeutic delivery
Abstract
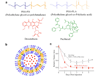
A growing number of medications must be administered through parenteral delivery, i.e., intravenous, intramuscular, or subcutaneous injection, to ensure effectiveness of the therapeutic. For some therapeutics, the use of delivery vehicles in conjunction with this delivery mechanism can improve drug efficacy and patient compliance. Macromolecular self-assembly has been exploited recently to engineer materials for the encapsulation and controlled delivery of therapeutics. Self-assembled materials offer the advantages of conventional crosslinked materials normally used for release, but also provide the ability to tailor specific bulk material properties, such as release profiles, at the molecular level via monomer design. As a result, the design of materials from the "bottom up" approach has generated a variety of supramolecular devices for biomedical applications. This review provides an overview of self-assembling molecules, their resultant structures, and their use in therapeutic delivery. It highlights the current progress in the design of polymer- and peptide-based self-assembled materials.
Figures





References
-
- Reichert JM, Wenger JB. Development trends for new cancer therapeutics and vaccines. Drug Discov Today. 2008;13:30–37. - PubMed
-
- Chaubal MV, Roseman TJ. Drug delivery trends for parenteral therapeutics. Drug Deliv Syst. 2006;21:388–397.
-
- Pawar R, Ben-Ari A, Domb AJ. Protein and peptide parenteral controlled delivery. Exp Opin Biol Ther. 2004;4:1203–1212. - PubMed
-
- Sagar GH, Arunagirinathan MA, Bellare JR. Self-assembled surfactant nano-structures important in drug delivery: a review. Indian J Exp Biol. 2007;45:133–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

