Experimental analysis of the rice mitochondrial proteome, its biogenesis, and heterogeneity
- PMID: 19010998
- PMCID: PMC2633852
- DOI: 10.1104/pp.108.131300
Experimental analysis of the rice mitochondrial proteome, its biogenesis, and heterogeneity
Abstract
Mitochondria in rice (Oryza sativa) are vital in expanding our understanding of the cellular response to reoxygenation of tissues after anaerobiosis, the crossroads of carbon and nitrogen metabolism, and the role of respiratory energy generation in cytoplasmic male sterility. We have combined density gradient and surface charge purification techniques with proteomics to provide an in-depth proteome of rice shoot mitochondria covering both soluble and integral membrane proteins. Quantitative comparisons of mitochondria purified by density gradients and after further surface charge purification have been used to ensure that the proteins identified copurify with mitochondria and to remove contaminants from the analysis. This rigorous approach to defining a subcellular proteome has yielded 322 nonredundant rice proteins and highlighted contaminants in previously reported rice mitochondrial proteomes. Comparative analysis with the Arabidopsis (Arabidopsis thaliana) mitochondrial proteome reveals conservation of a broad range of known and unknown function proteins in plant mitochondria, with only approximately 20% not having a clear homolog in the Arabidopsis mitochondrial proteome. As in Arabidopsis, only approximately 60% of the rice mitochondrial proteome is predictable using current organelle-targeting prediction tools. Use of the rice protein data set to explore rice transcript data provided insights into rice mitochondrial biogenesis during seed germination, leaf development, and heterogeneity in the expression of nucleus-encoded mitochondrial components in different rice tissues. Highlights include the identification of components involved in thiamine synthesis, evidence for coexpressed and unregulated expression of specific components of protein complexes, a selective anther-enhanced subclass of the decarboxylating segment of the tricarboxylic acid cycle, the differential expression of DNA and RNA replication components, and enhanced expression of specific metabolic components in photosynthetic tissues.
Figures


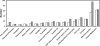


References
-
- Adams KL, Daley DO, Qiu YL, Whelan J, Palmer J (2000) Repeated, recent and diverse transfers of a mitochondrial gene to the nucleus in flowering plants. Nature 408 354–357 - PubMed
-
- Baerenfaller K, Grossmann J, Grobei MA, Hull R, Hirsch-Hoffmann M, Yalovsky S, Zimmermann P, Grossniklaus U, Gruissem W, Baginsky S (2008) Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science 320 938–941 - PubMed
-
- Bardel J, Louwagie M, Jaquinod M, Jourdain A, Luche S, Rabilloud T, Macherel D, Garin J, Bourguignon J (2002) A survey of the plant mitochondrial proteome in relation to development. Proteomics 2 880–898 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

