CTGF is increased in basal deposits and regulates matrix production through the ERK (p42/p44mapk) MAPK and the p38 MAPK signaling pathways
- PMID: 19011018
- PMCID: PMC2729056
- DOI: 10.1167/iovs.08-2383
CTGF is increased in basal deposits and regulates matrix production through the ERK (p42/p44mapk) MAPK and the p38 MAPK signaling pathways
Abstract
Purpose: Matrix expansion is an early change in age-related maculopathy. The aim of this study was to determine whether connective tissue growth factor (CTGF) regulates the production of extracellular matrix components by retinal pigmented epithelial (RPE) cells.
Methods: ARPE-19 cells were treated with CTGF and analyzed for fibronectin, laminin, and MMP-2 by RT-qPCR, Western blot analysis, or zymography. Cells were also pretreated with an MEK-1/2 inhibitor (PD98059) or a p38 inhibitor (SB203580) and an anti-CTGF antibody to analyze the signaling contributing to fibronectin, laminin, and MMP-2 production. Human maculas were analyzed for mRNA using laser capture microdissected RPE cells and by immunohistochemistry for the topographic distribution of CTGF.
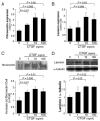
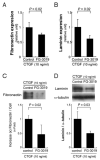
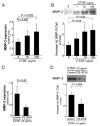
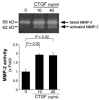
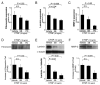
Results: CTGF induced fibronectin mRNA (P=0.006) and protein (P=0.006), and laminin mRNA (P=0.006) and protein (P=0.02) by ARPE-19 cells. CTGF also induced MMP-2 mRNA (P=0.002) and protein secretion (P=0.04). Using zymography, CTGF increased the latent and active forms of MMP-2 compared to controls (P=0.02). An anti-CTGF antibody inhibited fibronectin, laminin, and MMP-2 after CTGF stimulation. CTGF increased the phosphorylation of p38 and ERK1/2. Fibronectin and MMP-2 mRNA and protein were suppressed by a MEK-1/2 inhibitor, but not with a p38 inhibitor. Laminin expression was suppressed by both inhibitors. RT-qPCR analysis showed that macular RPE cells from human donors express CTGF. Immunohistochemistry of human maculas showed strong labeling of CTGF in Bruch membrane, including basal deposits and drusen.
Conclusions: CTGF is increased in basal deposits and drusen of AMD specimens, and it induces matrix protein production in ARPE-19 cells through the ERK (p42/p44(mapk)) and p38(mapk) signaling pathways.
Conflict of interest statement
Disclosure:
Figures

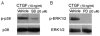

References
-
- Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. - PubMed
-
- van der Schaft TL, Mooy CM, de Bruijn WC, Bosman FT, de Jong PT. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232:40–46. - PubMed
-
- Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339. - PubMed
-
- Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006;47:729–737. - PubMed
-
- An E, Lu X, Flippin J, et al. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J Proteome Res. 2006;5:2599–2610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

