Nanoparticle-induced surface reconstruction of phospholipid membranes
- PMID: 19011086
- PMCID: PMC2587577
- DOI: 10.1073/pnas.0807296105
Nanoparticle-induced surface reconstruction of phospholipid membranes
Abstract
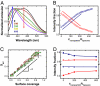
The nonspecific adsorption of charged nanoparticles onto single-component phospholipid bilayers bearing phosphocholine headgroups is shown, from fluorescence and calorimetry experiments, to cause surface reconstruction at the points where nanoparticles adsorb. Nanoparticles of negative charge induce local gelation in otherwise fluid bilayers; nanoparticles of positive charge induce otherwise gelled membranes to fluidize locally. Through this mechanism, the phase state deviates from the nominal phase transition temperature by tens of degrees. This work generalizes the notions of environmentally induced surface reconstruction, prominent in metals and semiconductors. Bearing in mind that chemical composition in these single-component lipid bilayers is the same everywhere, this offers a mechanism to generate patchy functional properties in phospholipid membranes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Groves JT, Boxer SG. Micropattern formation in supported lipid membranes. Acc Chem Res. 2002;35:149–157. - PubMed
-
- Daniel S, Albertorio F, Cremer PS. Making lipid membranes rough, tough and ready to hit the road. MRS Bull. 2006;31:536–540.
-
- Römer W, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. - PubMed
-
- Fielding CJ, editor. Lipid Rafts and Caveolae. Weinheim, Germany: Wiley-VCH; 2006.
-
- Anderson RG, Jacobson KA. Role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

