Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea
- PMID: 19011097
- PMCID: PMC2587543
- DOI: 10.1073/pnas.0808175105
Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea
Abstract
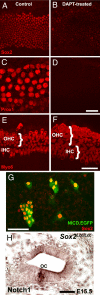
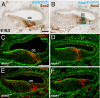
Sox2 is a high-mobility transcription factor that is one of the earliest markers of developing inner ear prosensory domains. In humans, mutations in SOX2 cause sensorineural hearing loss and a loss of function study in mice showed that Sox2 is required for prosensory formation in the cochlea. However, the specific roles of Sox2 have not been determined. Here we illustrate a dynamic role of Sox2 as an early permissive factor in prosensory domain formation followed by a mutually antagonistic relationship with Atoh1, a bHLH protein necessary for hair cell development. We demonstrate that decreased levels of Sox2 result in precocious hair cell differentiation and an over production of inner hair cells and that these effects are likely mediated through an antagonistic interaction between Sox2 and the bHLH molecule Atoh1. Using gain- and loss-of-function experiments we provide evidence for the molecular pathway responsible for the formation of the cochlear prosensory domain. Sox2 expression is promoted by Notch signaling and Prox1, a homeobox transcription factor, is a downstream target of Sox2. These results demonstrate crucial and diverse roles for Sox2 in the development, specification, and maintenance of sensory cells within the cochlea.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. - PubMed
-
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. - PubMed
-
- Kiernan AE, et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. - PubMed
-
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

