Neutral evolution in paroxysmal nocturnal hemoglobinuria
- PMID: 19011109
- PMCID: PMC2587638
- DOI: 10.1073/pnas.0802749105
Neutral evolution in paroxysmal nocturnal hemoglobinuria
Abstract
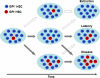
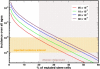
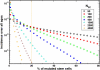
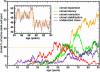
Paroxysmal nocturnal hemoglobinuria is an acquired hematopoietic stem cell (HSC) disorder characterized by the partial or complete deficiency of glycosyl-phosphatidylinositol (GPI)-linked membrane proteins, which leads to intravascular hemolysis. A loss of function mutation in the PIG-A gene, required for GPI biosynthesis, explains how the deficiency of many membrane proteins can result from a single genetic event. However, to date the mechanism of expansion of the GPI(-) clone has not been fully understood. Two hypotheses have been proposed: A selective advantage of GPI(-) cells because of a second mutation or a conditional growth advantage of GPI(-) cells in the presence of an immune attack on normal (GPI(+)) HSCs. Here, we explore a third possibility, whereby the PNH clone does not have a selective advantage. Simulations in a large virtual population accurately reproduce the known incidence of the disease; and the fit is optimized when the number of stem cells is decreased, reflecting a component of bone marrow failure in PNH. The model also accounts for the occurrence of spontaneous cure in PNH, consequent on clonal extinction. Thus, a clonal advantage may not be always necessary to explain clonal expansion in PNH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Luzzatto L, Bessler M, Rotoli B. Somatic mutations in paroxysmal nocturnal hemoglobinuria: A blessing in disguise? Cell. 1997;88:1–4. - PubMed
-
- Hillmen P, Bessler M, Mason PJ, Watkins WM, Luzzatto L. Specific defect in N-acetylglucosamine incorporation in the biosynthesis of the glycosylphosphatidylinositol anchor in cloned cell lines from patients with paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci USA. 1993;90:5272–5276. - PMC - PubMed
-
- Miyata T, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330:249–255. - PubMed
-
- Rotoli B, Luzzatto L. Paroxysmal nocturnal hemoglobinuria. Semin Hematol. 1989;26:201–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

