Protein repair L-isoaspartyl methyltransferase 1 is involved in both seed longevity and germination vigor in Arabidopsis
- PMID: 19011119
- PMCID: PMC2613667
- DOI: 10.1105/tpc.108.058479
Protein repair L-isoaspartyl methyltransferase 1 is involved in both seed longevity and germination vigor in Arabidopsis
Abstract
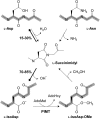
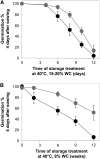
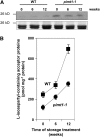
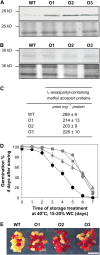
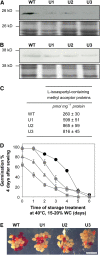
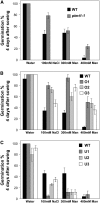
The formation of abnormal amino acid residues is a major source of spontaneous age-related protein damage in cells. The protein l-isoaspartyl methyltransferase (PIMT) combats protein misfolding resulting from l-isoaspartyl formation by catalyzing the conversion of abnormal l-isoaspartyl residues to their normal l-aspartyl forms. In this way, the PIMT repair enzyme system contributes to longevity and survival in bacterial and animal kingdoms. Despite the discovery of PIMT activity in plants two decades ago, the role of this enzyme during plant stress adaptation and in seed longevity remains undefined. In this work, we have isolated Arabidopsis thaliana lines exhibiting altered expression of PIMT1, one of the two genes encoding the PIMT enzyme in Arabidopsis. PIMT1 overaccumulation reduced the accumulation of l-isoaspartyl residues in seed proteins and increased both seed longevity and germination vigor. Conversely, reduced PIMT1 accumulation was associated with an increase in the accumulation of l-isoaspartyl residues in the proteome of freshly harvested dry mature seeds, thus leading to heightened sensitivity to aging treatments and loss of seed vigor under stressful germination conditions. These data implicate PIMT1 as a major endogenous factor that limits abnormal l-isoaspartyl accumulation in seed proteins, thereby improving seed traits such as longevity and vigor. The PIMT repair pathway likely works in concert with other anti-aging pathways to actively eliminate deleterious protein products, thus enabling successful seedling establishment and strengthening plant proliferation in natural environments.
Figures

References
-
- Association of Official Seed Analysts (1983). Seed Vigor Testing Handbook, Contribution No. 32. (Lincoln, Nebraska: Association of Official Seed Analysts).
-
- Aswad, D.W., Paranandi, M.V., and Schurter, B.T. (2000). Isoaspartate in peptides and proteins: Formation, significance, and analysis. J. Pharm. Biomed. Anal. 21 1129–1136. - PubMed
-
- Athmer, L., Kindrachuk, J., Georges, F., and Napper, S. (2002). The influence of protein structure on the products emerging from succinimide hydrolysis. J. Biol. Chem. 277 30502–30507. - PubMed
-
- Bailly, C. (2004). Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 14 93–107.
-
- Bailly, C., El-Maarouf-Bouteau, H., and Corbineau, F. (2008). From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. C. R. Biol. 331 806–814. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

