Cigarette smoke induces cellular senescence via Werner's syndrome protein down-regulation
- PMID: 19011155
- PMCID: PMC2643077
- DOI: 10.1164/rccm.200802-320OC
Cigarette smoke induces cellular senescence via Werner's syndrome protein down-regulation
Abstract
Rationale: Werner's syndrome is a genetic disorder that causes premature aging due to loss-of-function mutations in a gene encoding a member of the RecQ helicase family. Both Werner's syndrome and cigarette smoking accelerate aging. No studies have examined the effect of cigarette smoke on Werner's syndrome protein.
Objectives: To investigate the role of Werner's syndrome protein in cigarette smoke-induced cellular senescence.
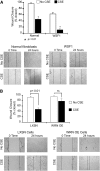
Methods: Cellular senescence and amounts of Werner's syndrome protein were measured in fibroblasts isolated from patients with emphysema and compared with age-matched nonsmokers. The in vitro effects of cigarette smoke on amounts of Werner's syndrome protein, function, and senescence were also evaluated in primary human lung fibroblasts and epithelial cells.
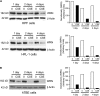
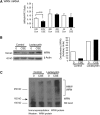
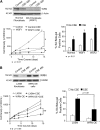
Measurements and main results: Cultured lung fibroblasts isolated from patients with emphysema exhibited a senescent phenotype accompanied by a decrease in Werner's syndrome protein. Cigarette smoke extract decreased Werner's syndrome protein in cultured fibroblasts and epithelial cells. Werner's syndrome protein-deficient fibroblasts were more susceptible to cigarette smoke-induced cellular senescence and cell migration impairment. In contrast, exogenous overexpression of Werner's syndrome protein attenuated the cigarette smoke effects.
Conclusions: Cigarette smoke induces cellular senescence and cell migration impairment via Werner's syndrome protein down-regulation. Rescue of Werner's syndrome protein down-regulation may represent a potential therapeutic target for smoking-related diseases.
Figures



References
-
- Martin GM. Genetic syndromes in man with potential relevance to the pathobiology of aging. Birth Defects Orig Artic Ser 1978;14:5–39. - PubMed
-
- Epstein CJ, Martin GM, Schultz AL, Motulsky AG. Werner's syndrome: a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 1966;45:177–221. - PubMed
-
- Goto M, Tanimoto K, Horiuchi Y, Sasazuki T. Family analysis of Werner's syndrome: a survey of 42 Japanese families with a review of the literature. Clin Genet 1981;19:8–15. - PubMed
-
- Brown WT, Kieras FJ, Houck GE Jr, Dutkowski R, Jenkins EC. A comparison of adult and childhood progerias: Werner syndrome and Hutchinson-Gilford progeria syndrome. Adv Exp Med Biol 1985;190:229–244. - PubMed
-
- Martin GM, Oshima J, Gray MD, Poot M. What geriatricians should know about the Werner syndrome. J Am Geriatr Soc 1999;47:1136–1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

