Highly efficient molybdenum-based catalysts for enantioselective alkene metathesis
- PMID: 19011612
- PMCID: PMC2663850
- DOI: 10.1038/nature07594
Highly efficient molybdenum-based catalysts for enantioselective alkene metathesis
Abstract
Discovery of efficient catalysts is one of the most compelling objectives of modern chemistry. Chiral catalysts are in particularly high demand, as they facilitate synthesis of enantiomerically enriched small molecules that are critical to developments in medicine, biology and materials science. Especially noteworthy are catalysts that promote-with otherwise inaccessible efficiency and selectivity levels-reactions demonstrated to be of great utility in chemical synthesis. Here we report a class of chiral catalysts that initiate alkene metathesis with very high efficiency and enantioselectivity. Such attributes arise from structural fluxionality of the chiral catalysts and the central role that enhanced electronic factors have in the catalytic cycle. The new catalysts have a stereogenic metal centre and carry only monodentate ligands; the molybdenum-based complexes are prepared stereoselectively by a ligand exchange process involving an enantiomerically pure aryloxide, a class of ligands scarcely used in enantioselective catalysis. We demonstrate the application of the new catalysts in an enantioselective synthesis of the Aspidosperma alkaloid, quebrachamine, through an alkene metathesis reaction that cannot be promoted by any of the previously reported chiral catalysts.
Figures

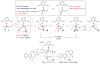

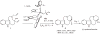
Comment in
-
Organometallic chemistry: catalyst takes control to heart.Nature. 2008 Dec 18;456(7224):883-5. doi: 10.1038/456883a. Nature. 2008. PMID: 19092919 No abstract available.
References
-
- Hoveyda AH, Zhugralin AR. The remarkable metal-catalysed olefin metathesis reaction. Nature. 2007;450:243–251. - PubMed
-
- Hashimoto S, Komeshima N, Koga K. Asymmetric Diels–Alder reaction catalysed by chiral alkoxyaluminum dichloride. J Chem Soc Chem Commun. 1979:437–438.
-
- Tayama E, Saito A, Ooi T, Maruoka K. Activation of ether functionality of allyl vinyl ethers by chiral bis(organoaluminum) Lewis acids: application to asymmetric Claisen rearrangement. Tetrahedron. 2002;58:8307–8312.
-
- Solans-Monfort X, Clot E, Copéret C, Eisenstein O. dO-Re-based olefin metathesis catalysts, Re(≡CR)(=CHR)(X)(Y): the key role of X and Y ligands for efficient active sites. J Am Chem Soc. 2005;127:14015–14025. - PubMed
-
- Poater A, Solans-Monfort X, Clot E, Copéret C, Eisenstein O. Understanding dO-olefin metathesis catalysts: Which metal, which ligands? J Am Chem Soc. 2007;129:8207–8216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

