Design, synthesis, and evaluation of tricyclic, conformationally constrained small-molecule mimetics of second mitochondria-derived activator of caspases
- PMID: 19012392
- PMCID: PMC2662380
- DOI: 10.1021/jm801146d
Design, synthesis, and evaluation of tricyclic, conformationally constrained small-molecule mimetics of second mitochondria-derived activator of caspases
Abstract
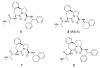
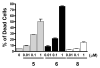
A series of tricyclic, conformationally constrained Smac mimetics have been designed, synthesized, and evaluated. The most potent compound 6 (WS-5) binds to XIAP, cIAP-1, and cIAP-2 with K(i) of 18, 1.1, and 4.2 nM, respectively. Compound 6 antagonizes XIAP in a functional assay, induces cIAP-1 degradation, inhibits cell growth with an IC(50) of 68 nM in the MDA-MB-231 cancer cell line, and effectively induces cancer cells to undergo apoptosis.
Figures





References
-
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95. - PubMed
-
- Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. - PubMed
-
- Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;1:239–252. - PubMed
-
- Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. - PubMed
-
- Holcik M, Gibson H, Korneluk RG. XIAP: Apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

