Proteomic analysis of RCL2 paraffin-embedded tissues
- PMID: 19012729
- PMCID: PMC4506168
- DOI: 10.1111/j.1582-4934.2008.00186.x
Proteomic analysis of RCL2 paraffin-embedded tissues
Abstract
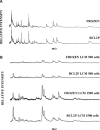
Histopathological diagnosis in most of the world's hospitals is based upon formalin-fixed and paraffin-embedded (FFPE) tissues. Although this standard fixation and embedding procedure keeps the tissue in excellent form for morphological and immunohistological analysis, FFPE is inappropriate for nucleic acids and protein studies. We investigated the potential value of RCL2, a new non-toxic fixative, for sparing proteins preserved in paraffin-embedded tissues. Normal colonic mucosa tissue was fixed in RCL2 prior to paraffin embedding (RCL2P), and then processed for quality and quantity of protein conservation, as compared to frozen and FFPE tissues using complementary proteomic analysis approaches. Using 4 different protein extraction protocols, RCL2P tissue consistently showed the highest protein yield. Similar protein patterns were observed with RCL2P and frozen tissues using mono and bi-dimensional electrophoresis. Moreover, membrane, cytoplasmic and nuclear proteins, as well as phosphorylated proteins, were successfully detected using western-blot. Furthermore, protein patterns observed by mass spectrometry analysis after laser-captured microdissection were found to be identical for frozen and RCL2-fixed tissues. At last, immunohistochemistry using various antibodies showed comparable results between both tissue storage methods. We concluded that RCL2 has great potential for performing both morphological and molecular analyses on the same archival paraffin-embedded tissue sample, and can be a new method for investigating protein biomarkers.
Figures





References
-
- Lehmann U, Kreipe H. Real-time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Method. 2001;25:409–18. - PubMed
-
- Becker KF, Schott C, Hipp S, Metzger V, Porschewski P, Beck R, Nahrig J, Becker I, Hofler H. Quantitative protein analysis from formalin-fixed tissues: implications for translational clinical research and nanoscale molecular diagnosis. J Pathol. 2007;211:370–8. - PubMed
-
- Crockett DK. Lin Z. Vaughn CP. Lim MS, Elenitoba-Johnson KS. Identification of proteins from formalin-fixed paraffin-embedded cells by LC-MS/MS. Lab Invest. 2005;85:1405–15. - PubMed
-
- Hwang SI, Thumar J, Lundgren DH, Rezaul K, Mayya V, Wu L, Eng J, Wright ME, Han DK. Direct cancer tissue pro-teomics: a method to identify candidate cancer biomarkers from formalin-fixed paraffin-embedded archival tissues. Oncogene. 2007;26:65–76. - PubMed
-
- Palmer-Toy DE, Krastins B, Sarracino DA, Nadol JB, Jr, Merchant SN. Efficient method for the proteomic analysis of fixed and embedded tissues. J Proteome Res. 2005;4:2404–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

